Emergence and significance of carbohydrate-specific antibodies
- PMID: 32753697
- PMCID: PMC7449879
- DOI: 10.1038/s41435-020-0105-9
Emergence and significance of carbohydrate-specific antibodies
Abstract
Carbohydrate-specific antibodies are widespread among all classes of immunoglobulins. Despite their broad occurrence, little is known about their formation and biological significance. Carbohydrate-specific antibodies are often classified as natural antibodies under the assumption that they arise without prior exposure to exogenous antigens. On the other hand, various carbohydrate-specific antibodies, including antibodies to ABO blood group antigens, emerge after the contact of immune cells with the intestinal microbiota, which expresses a vast diversity of carbohydrate antigens. Here we explore the development of carbohydrate-specific antibodies in humans, addressing the definition of natural antibodies and the production of carbohydrate-specific antibodies upon antigen stimulation. We focus on the significance of the intestinal microbiota in shaping carbohydrate-specific antibodies not just in the gut, but also in the blood circulation. The structural similarity between bacterial carbohydrate antigens and surface glycoconjugates of protists, fungi and animals leads to the production of carbohydrate-specific antibodies protective against a broad range of pathogens. Mimicry between bacterial and human glycoconjugates, however, can also lead to the generation of carbohydrate-specific antibodies that cross-react with human antigens, thereby contributing to the development of autoimmune disorders.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
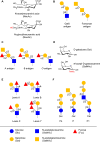

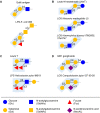
References
-
- Freeze HH, Hart GW, Schnaar RL Glycosylation Precursors. In: Varki ACR, Esko JD, et al., editor Essentials of glycobiology. 3rd ed., vol. Chapter 5. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

