Engineering Thermo-pH Dual Responsive Hydrogel for Enhanced Tumor Accumulation, Penetration, and Chemo-Protein Combination Therapy
- PMID: 32753862
- PMCID: PMC7342477
- DOI: 10.2147/IJN.S253990
Engineering Thermo-pH Dual Responsive Hydrogel for Enhanced Tumor Accumulation, Penetration, and Chemo-Protein Combination Therapy
Abstract
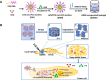
Purpose: Combined chemotherapeutic drug and protein drug has been a widely employed strategy for tumor treatment. To realize both tumor accumulation and deep tumor penetration for drugs with different pharmacokinetics, we propose a structure-transformable, thermo-pH dual responsive co-delivery system to co-load granzyme B/docetaxel (GrB/DTX).
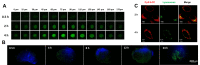
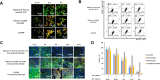
Methods: Thermo-sensitive hydrogels based on diblock copolymers (mPEG-b-PELG) were synthesized through ring opening polymerization. GrB/DTX mini micelles (GDM) was developed by co-loading these two drugs in pH-sensitive mini micelles, and the GDM-incorporated thermo-sensitive hydrogel (GDMH) was constructed. The thermo-induced gelation behavior of diblock copolymers and the physiochemical properties of GDMH were characterized. GDMH degradation and deep tumor penetration of released mini micelles were confirmed. The pH-sensitive disassembly and lysosomal escape abilities of released mini micelles were evaluated. In vitro cytotoxicity was studied using MTT assays and the in vivo antitumor efficacy study was evaluated in B16-bearing C57BL/6 mice.
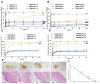
Results: GDMH was gelatinized at body temperature and can be degraded by proteinase to release mini micelles. The mini micelles incorporated in GDMH can achieve deep tumor penetration and escape from lysosomes to release GrB and DTX. MTT results showed that maximum synergistic antitumor efficacy of GrB and DTX was observed at mass ratio of 1:100. Our in vivo antitumor efficacy study showed that GDMH inhibited tumor growth in the subcutaneous tumor model and in the post-surgical recurrence model.
Conclusion: The smart-designed transformable GDMH can facilitate tumor accumulation, deep tumor penetration, and rapid drug release to achieve synergistic chemo-protein therapy.
Keywords: chemo-protein combination therapy; hydrogel; structure-transformable; thermo-pH dual responsive.
© 2020 Pang et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

