Effects of Trigger Point Dry Needling on Neuromuscular Performance and Pain of Individuals Affected by Patellofemoral Pain: A Randomized Controlled Trial
- PMID: 32753943
- PMCID: PMC7354013
- DOI: 10.2147/JPR.S240376
Effects of Trigger Point Dry Needling on Neuromuscular Performance and Pain of Individuals Affected by Patellofemoral Pain: A Randomized Controlled Trial
Erratum in
-
Erratum: Effects of Trigger Point Dry Needling on Neuromuscular Performance and Pain of Individuals Affected by Patellofemoral Pain: A Randomized Controlled Trial [Corrigendum].J Pain Res. 2020 Sep 7;13:2237. doi: 10.2147/JPR.S278493. eCollection 2020. J Pain Res. 2020. PMID: 32982385 Free PMC article.
Abstract
Purpose: To investigate the effects of trigger point dry needling (TrP-DN) on exercise-induced patellofemoral pain syndrome (PFPS).
Patients and methods: In this randomized, single-blind, parallel-group trial, 50 patients with PFPS were randomly allocated to the following two groups: the TrP-DN group (n = 25) and the Sham needling group (n = 25). Patients in both groups were asked to perform a stretching exercise of the quadriceps daily after needling. The needling group received a single session of TrP-DN to trigger points (TrPs) in the vastus medialis oblique (VMO), vastus lateralis (VL), and rectus femoris muscles (once a week for 6 weeks), and the Sham group received placebo needling. Visual analogue scale (VAS) for pain intensity and Kujala questionnaire for the functional status were assessed before treatment, 3 and 6 weeks after treatment, and at the 3-month follow-up. The ratio of the myoelectric amplitude of the vastus medialis oblique and vastus lateralis muscles (VMO/VL) was assessed before treatment and 6 weeks after treatment.
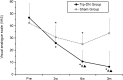
Results: There was no significant difference in the general data between the two groups. The VAS scores and Kujala scores in the TrP-DN group were significantly improved and increased at the 3-week treatment visit, 6-week treatment visit, and 3-month follow-up compared to the scores before treatment; and the scores in the Sham group were only significantly improved at the 3-week treatment visit, and 6-week treatment visit. VAS scores in the TrP-DN group were significantly lower and Kujala scores were significantly higher at the 6-week treatment visit and the 3-month follow-up compared to those in the Sham group. The VMO/VL ratio in the TrP-DN group was significantly increased at the 6-week treatment visit compared to that before treatment.
Conclusion: TrP-DN at the quadriceps combined with stretch can reduce the pain, and improves the clinical symptoms and function, the VMO/VL ratio, and the coordination of VMO and VL in patients with PFPS.
Keywords: myofascial trigger points; patellofemoral pain syndrome; stretching.
© 2020 Ma et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


References
-
- Crossley KM, Stefanik JJ, Selfe J, et al. Patellofemoral pain consensus statement from the 4th international patellofemoral pain research retreat, manchester. Part 1: terminology, definitions, clinical examination, natural history, patellofemoral osteoarthritis and patient-reported outcome measures. Br J Sports Med. 2016;50:839–843. doi:10.1136/bjsports-2016-096384 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Medical

