Semantic Processing in Autism Spectrum Disorders Is Associated With the Timing of Language Acquisition: A Magnetoencephalographic Study
- PMID: 32754020
- PMCID: PMC7366733
- DOI: 10.3389/fnhum.2020.00267
Semantic Processing in Autism Spectrum Disorders Is Associated With the Timing of Language Acquisition: A Magnetoencephalographic Study
Abstract
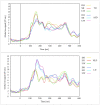
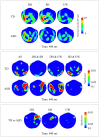
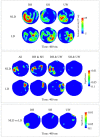
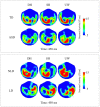
Individuals with autism show difficulties in using sentence context to identify the correct meaning of ambiguous words, such as homonyms. In this study, the brain basis of sentence context effects on word understanding during reading was examined in autism spectrum disorder (ASD) and typical development (TD) using magnetoencephalography. The correlates of a history of developmental language delay in ASD were also investigated. Event related field responses at early (150 ms after the onset of a final word) and N400 latencies are reported for three different types of sentence final words: dominant homonyms, subordinate homonyms, and unambiguous words. Clear evidence for semantic access was found at both early and conventional N400 latencies in both TD participants and individuals with ASD with no history of language delay. By contrast, modulation of evoked activity related to semantic access was weak and not significant at early latencies in individuals with ASD with a history of language delay. The reduced sensitivity to semantic context in individuals with ASD and language delay was accompanied by strong right hemisphere lateralization at early and N400 latencies; such strong activity was not observed in TD individuals and individuals with ASD without a history of language delay at either latency. These results provide new evidence and support for differential neural mechanisms underlying semantic processing in ASD, and indicate that delayed language acquisition in ASD is associated with different lateralization and processing of language.
Keywords: N400; autism; language; magnetoencephalography; semantics.
Copyright © 2020 Ahtam, Braeutigam and Bailey.
Figures


References
-
- Ahtam B. (2009). A Magnetoencephalographic Study of Contextual Effects on Semantic Processing in Autism Spectrum Disorders. Ph. D. Thesis, University of Oxford, Oxford.
-
- American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders (DSM-V). Washington, DC: American Psychiatric Association.
-
- Baayen R. H., Piepenbrock R., Gulikers L. (1995). The CELEX Lexical Database. Philadelphia, PA: Linguistic Data Consortium.
-
- Bailey A. J., Braeutigam S., Swithenby S. J. (2000). “Context and reading: A MEG study of autistic adults,” in Proceedings of the 12th International Conference on Biomagnetism, (Espoo: Helsinki University of Technology; ).
LinkOut - more resources
Full Text Sources

