Four Chromosomal Type IV Secretion Systems in Helicobacter pylori: Composition, Structure and Function
- PMID: 32754140
- PMCID: PMC7366825
- DOI: 10.3389/fmicb.2020.01592
Four Chromosomal Type IV Secretion Systems in Helicobacter pylori: Composition, Structure and Function
Abstract
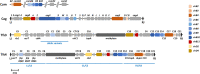
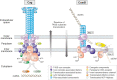
The pathogenic bacterium Helicobacter pylori is genetically highly diverse and a major risk factor for the development of peptic ulcer disease and gastric adenocarcinoma in humans. During evolution, H. pylori has acquired multiple type IV secretion systems (T4SSs), and then adapted for various purposes. These T4SSs represent remarkable molecular transporter machines, often associated with an extracellular pilus structure present in many bacteria, which are commonly composed of multiple structural proteins spanning the inner and outer membranes. By definition, these T4SSs exhibit central functions mediated through the contact-dependent conjugative transfer of mobile DNA elements, the contact-independent release and uptake of DNA into and from the extracellular environment as well as the secretion of effector proteins in mammalian host target cells. In recent years, numerous features on the molecular functionality of these T4SSs were disclosed. H. pylori encodes up to four T4SSs on its chromosome, namely the Cag T4SS present in the cag pathogenicity island (cagPAI), the ComB system, as well as the Tfs3 and Tfs4 T4SSs, some of which exhibit unique T4SS functions. The Cag T4SS facilitates the delivery of the CagA effector protein and pro-inflammatory signal transduction through translocated ADP-heptose and chromosomal DNA, while various structural pilus proteins can target host cell receptors such as integrins or TLR5. The ComB apparatus mediates the import of free DNA from the extracellular milieu, whereas Tfs3 may accomplish the secretion or translocation of effector protein CtkA. Both Tfs3 and Tfs4 are furthermore presumed to act as conjugative DNA transfer machineries due to the presence of tyrosine recombinases with cognate recognition sequences, conjugational relaxases, and potential origins of transfer (oriT) found within the tfs3 and tfs4 genome islands. In addition, some extrachromosomal plasmids, transposons and phages have been discovered in multiple H. pylori isolates. The genetic exchange mediated by DNA mobilization events of chromosomal genes and plasmids combined with recombination events could account for much of the genetic diversity found in H. pylori. In this review, we highlight our current knowledge on the four T4SSs and the involved mechanisms with consequences for H. pylori adaptation to the hostile environment in the human stomach.
Keywords: DNA transfer; cag; competence; conjugation; pathogenicity island; recombination; type IV secretion system; virulence.
Copyright © 2020 Fischer, Tegtmeyer, Stingl and Backert.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

