Calcium Supplement by Tetracycline guided amorphous Calcium Carbonate potentiates Osteoblast promotion for Synergetic Osteoporosis Therapy
- PMID: 32754265
- PMCID: PMC7392017
- DOI: 10.7150/thno.45142
Calcium Supplement by Tetracycline guided amorphous Calcium Carbonate potentiates Osteoblast promotion for Synergetic Osteoporosis Therapy
Abstract
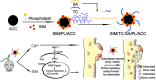
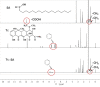
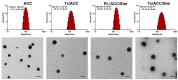
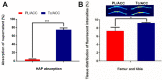
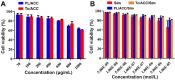
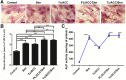
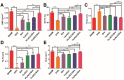
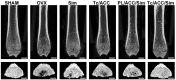
Background: The calcium supplement is a clinically approved approach for osteoporosis therapy but usually requires a large dosage without targetability and with poor outcome. This modality is not fully explored in current osteoporosis therapy due to the lack of proper calcium supplement carrier. Methods: In this study, we constructed a tetracycline (Tc) modified and simvastatin (Sim) loaded phospholipid-amorphous calcium carbonate (ACC) hybrid nanoparticle (Tc/ACC/Sim). Results: The resulted Tc/ACC/Sim was able to enhance its accumulation at the osteoporosis site. Most importantly, the combination of calcium supplement and Sim offered synergetic osteoblast promotion therapy of osteoporosis with advanced performance than non-targeted system or mono therapy. Conclusion: This platform provides an alternative approach to stimulate bone formation by synergetic promotion of osteoblast differentiation using calcium supplement and Sim.
Keywords: amorphous calcium carbonate; calcium supplement; osteoblast promotion; osteoporosis.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


Similar articles
-
Water/pH dual responsive in situ calcium supplement collaborates simvastatin for osteoblast promotion mediated osteoporosis therapy via oral medication.J Control Release. 2021 Jan 10;329:121-135. doi: 10.1016/j.jconrel.2020.11.059. Epub 2020 Dec 3. J Control Release. 2021. PMID: 33279604
-
Tetracycline-grafted PLGA nanoparticles as bone-targeting drug delivery system.Int J Nanomedicine. 2015 Sep 8;10:5671-85. doi: 10.2147/IJN.S88798. eCollection 2015. Int J Nanomedicine. 2015. PMID: 26388691 Free PMC article.
-
Bone-targeted delivery of simvastatin-loaded PEG-PLGA micelles conjugated with tetracycline for osteoporosis treatment.Drug Deliv Transl Res. 2018 Oct;8(5):1090-1102. doi: 10.1007/s13346-018-0561-1. Drug Deliv Transl Res. 2018. Retraction in: Drug Deliv Transl Res. 2024 May;14(5):1392. doi: 10.1007/s13346-023-01468-8. PMID: 30027372 Retracted.
-
Strontium ranelate in osteoporosis.Curr Pharm Des. 2002;8(21):1907-16. doi: 10.2174/1381612023393639. Curr Pharm Des. 2002. PMID: 12171530 Review.
-
Marine algae possess therapeutic potential for Ca-mineralization via osteoblastic differentiation.Adv Food Nutr Res. 2011;64:429-41. doi: 10.1016/B978-0-12-387669-0.00033-8. Adv Food Nutr Res. 2011. PMID: 22054966 Review.
Cited by
-
Amorphous calcium magnesium phosphate nanocomposites with superior osteogenic activity for bone regeneration.Regen Biomater. 2021 Nov 24;8(6):rbab068. doi: 10.1093/rb/rbab068. eCollection 2021 Dec. Regen Biomater. 2021. PMID: 34917396 Free PMC article.
-
An update on the advances in the field of nanostructured drug delivery systems for a variety of orthopedic applications.Drug Deliv. 2023 Dec;30(1):2241667. doi: 10.1080/10717544.2023.2241667. Epub 2023 Dec 1. Drug Deliv. 2023. PMID: 38037335 Free PMC article.
-
Temperature-Ion-pH Triple Responsive Gellan Gum as In Situ Hydrogel for Long-Acting Cancer Treatment.Gels. 2022 Aug 15;8(8):508. doi: 10.3390/gels8080508. Gels. 2022. PMID: 36005109 Free PMC article.
-
Unlocking the potential of amorphous calcium carbonate: A star ascending in the realm of biomedical application.Acta Pharm Sin B. 2024 Feb;14(2):602-622. doi: 10.1016/j.apsb.2023.08.027. Epub 2023 Sep 1. Acta Pharm Sin B. 2024. PMID: 38322345 Free PMC article. Review.
-
Fast shape memory function and personalized PLTMC/SIM/MBG composite scaffold for bone regeneration.Mater Today Bio. 2025 May 2;32:101791. doi: 10.1016/j.mtbio.2025.101791. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40416784 Free PMC article.
References
-
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025, J Bone Miner Res. 2007; 22: 465-75. - PubMed
-
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich G.A, Reginster JY. et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–41. - PubMed
-
- Klibanski A, Adams-Campbell L, Bassford TL, Blair SN, Boden SD, Dickersin K. et al. Osteoporosis prevention, diagnosis, and therapy. J Am Med Assoc. 2001;285:785–95.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

