LXR activation potentiates sorafenib sensitivity in HCC by activating microRNA-378a transcription
- PMID: 32754282
- PMCID: PMC7392029
- DOI: 10.7150/thno.45158
LXR activation potentiates sorafenib sensitivity in HCC by activating microRNA-378a transcription
Abstract
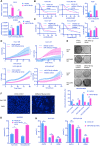
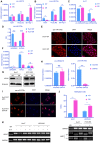
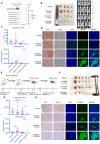
Sorafenib resistance is a major obstacle to the treatment of advanced hepatocellular carcinoma (HCC). MicroRNAs (miRNAs) are multifunctional regulators of gene expression with profound impact for human disease. Therefore, better understanding of the biological mechanisms of abnormally expressed miRNAs is critical to discovering novel, promising therapeutic targets for HCC treatment. This study aimed to investigate the role of miR-378a-3p in the sorafenib resistance of HCC and elucidate the underlying molecular mechanisms. Methods: A novel hub miR-378a-3p was identified based on miRNA microarray and bioinformatics analysis. The abnormal expression of miR-378-3p was validated in different HCC patient cohorts and sorafenib-resistant (SR) HCC cell lines. The functional role of miR-378a-3p and its downstream and upstream regulatory machinery were investigated by gain-of-function and loss-of-function assays in vitro and in vivo. Interactions among miR-378a-3p, LXRα, and IGF1R were examined by a series of molecular biology experiments. Then, the clinical relevance of miR-378a-3p and its targets were evaluated in HCC samples. HCC patient-derived xenograft (PDX) model was used to assess the therapeutic value of LXRα and its downstream miR-378a-3p. Results: miR-378a-3p expression was frequently reduced in established sorafenib-resistant HCC cell lines. The decreased miR-378a-3p levels correlated with poor overall survival of HCC patients following sorafenib treatment. miR-378a-3p overexpression induced apoptosis in SR HCC cells, whereas miR-378a-3p silencing exerted the opposite effects. IGF1R was identified as a novel target of miR-378a-3p. Furthermore, the primary miR-378 level was not consistent with its precursor miRNA level in SR HCC cells, which was attributed to the downregulation of exportin5 (XPO5) and subsequently reduced nuclear export of precursor miR-378 and restrained maturation of miR-378-3p. In this context, we combined an agonist GW3965 of liver X receptor alpha (LXRα), which functioned as a transcription activator of miRNA-378a, and its activation re-sensitized sorafenib-resistant cells to sorafenib treatment in vitro and in vivo. Conclusions: Our finding suggested decreased expression of XPO5 prevents maturation of miR-378a-3p, which leaded to the overexpression of IGF-1R and counteracted the effects of sorafenib-induced apoptosis. LXRα was able to activate miRNA-378a-3p transcription in HCC cells and could be a potential combinable treatment strategy with sorafenib to suppress HCC progression.
Keywords: IGF1R; LXRα; XPO5; hepatocellular carcinoma; microRNA; sorafenib resistance.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS. et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. - PubMed
-
- Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

