Engineered Hydrogels for Brain Tumor Culture and Therapy
- PMID: 32754347
- PMCID: PMC7402618
- DOI: 10.1007/s42242-020-00084-6
Engineered Hydrogels for Brain Tumor Culture and Therapy
Abstract
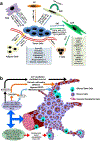
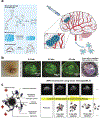
Brain tumors' severity ranges from benign to highly aggressive and invasive. Bioengineering tools can assist in understanding the pathophysiology of these tumors from outside the body and facilitate development of suitable antitumoral treatments. Here, we first describe the physiology and cellular composition of brain tumors. Then, we discuss the development of three-dimensional tissue models utilizing brain tumor cells. In particular, we highlight the role of hydrogels in providing a biomimetic support for the cells to grow into defined structures. Microscale technologies, such as electrospinning and bioprinting, and advanced cellular models aim to mimic the extracellular matrix and natural cellular localization in engineered tumor tissues. Lastly, we review current applications and prospects of hydrogels for therapeutic purposes, such as drug delivery and co-administration with other therapies. Through further development, hydrogels can serve as a reliable option for in vitro modeling and treatment of brain tumors for translational medicine.
Keywords: Brain tumor; bioengineering; cancer cells; drug delivery; hydrogel.
Figures




References
-
- Perkins A, Liu G (2016) Primary brain tumors in adults: diagnosis and treatment. Am Fam Physician 93 (3):211–217 - PubMed
-
- Puente P, Fettig N, Luderer MJ, Jin A, Shah S, Muz B, Kapoor V, Goddu SM, Salama NN, Tsien C, Thotala D, Shoghi K, Rogers B, Azab AK (2018) Injectable Hydrogels for Localized Chemotherapy and Radiotherapy in Brain Tumors. J Pharm Sci 107 (3):922–933. doi:10.1016/j.xphs.2017.10.042 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
