Multi-Step Unfolding and Rearrangement of α-Lactalbumin by SDS Revealed by Stopped-Flow SAXS
- PMID: 32754613
- PMCID: PMC7366515
- DOI: 10.3389/fmolb.2020.00125
Multi-Step Unfolding and Rearrangement of α-Lactalbumin by SDS Revealed by Stopped-Flow SAXS
Abstract
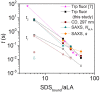
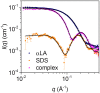
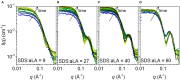
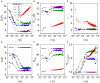
Interactions between proteins and surfactants are both of fundamental interest and relevant for applications in food, cosmetics and detergency. The anionic surfactant sodium dodecyl sulfate (SDS) denatures essentially all proteins. Denaturation typically involves a number of distinct steps where growing numbers of SDS molecules bind to the protein, as seen in multidisciplinary approaches combining several complementary techniques. We adopt this approach to study the SDS-induced unfolding of Ca2+-depleted α-lactalbumin (aLA), a protein particularly sensitive toward denaturation by surfactants. By combining stopped-flow mixing of protein and surfactant solutions with stopped-flow synchrotron small-angle X-ray scattering (SAXS), circular dichroism (CD) and Trp fluorescence, together with information from previous calorimetric studies, we construct a detailed picture of the unfolding process at the level of both protein and surfactant. A protein-surfactant complex is formed within the dead time of mixing (2.5 ms). Initially a cluster of SDS molecules binds asymmetrically, i.e., to one side of the protein, after which aLA redistributes around the SDS cluster. This occurs in two kinetic steps where the complex grows in number of both SDS and protein molecules, concomitant with protein unfolding. During these steps, the core-shell complex undergoes changes in shell thickness as well as core shape and radius. The entire process is very sensitive to SDS concentration and completes within 10 s at an SDS:aLA ratio of 9, decreasing to 0.2 s at 60 SDS:aLA. The number of aLA molecules per SDS complex drops from 1.9 to 1.0 over this range of ratios. While both CD and Trp kinetics reveal a fast and a slow conformational transition, only the slow transition is observed by SAXS, indicating that the protein-SDS complex (which is monitored by SAXS) adjusts to the presence of the unfolded protein. We attribute the rapid unfolding of aLA to its predominantly α-helical structure, which persists in SDS (albeit as isolated helices), enabling aLA to unfold without undergoing major secondary structural changes unlike β-sheet rich proteins. Nevertheless, the overall unfolding steps are broadly similar to those of the more β-rich protein β-lactoglobulin, suggesting that this unfolding model is representative of the general process of SDS-unfolding of proteins.
Keywords: circular dichroism; fluorescence; protein-SDS interactions; stopped-flow kinetics; supramolecular complex structures; synchrotron SAXS; unfolding mechanisms; α-lactalbumin.
Copyright © 2020 Jensen, Pedersen, Otzen and Pedersen.
Figures

References
-
- Burchard W., Kajiwara K., Whiffen D. H. (1970). The statistics of stiff chain molecules I. The particle scattering factor. Proc. R. S. Lond. Math. A Phys. Sci. 316, 185–199. 10.1098/rspa.1970.0074 - DOI
-
- Glatter O. (1977). A new method for the evaluation of small-angle scattering data. J. Appl. Crystall. 10, 415–421. 10.1107/S0021889877013879 - DOI
-
- Glatter O. (1979). The interpretation of real-space information from small-angle scattering experiments. J. Appl. Cryst. 12, 166–175. 10.1107/S0021889879012139 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

