Expression of inhibitory receptors by B cells in chronic human infectious diseases restricts responses to membrane-associated antigens
- PMID: 32754637
- PMCID: PMC7380957
- DOI: 10.1126/sciadv.aba6493
Expression of inhibitory receptors by B cells in chronic human infectious diseases restricts responses to membrane-associated antigens
Abstract
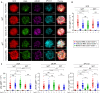
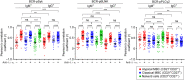
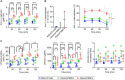
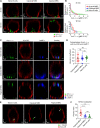
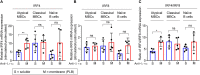
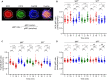
Chronic human infectious diseases, including malaria, are associated with a large expansion of a phenotypically and transcriptionally distinct subpopulation of B cells distinguished by their high expression of a variety of inhibitory receptors including FcγRIIB. Because these B cells, termed atypical memory B cells (MBCs), are unable to respond to soluble antigens, it was suggested that they contributed to the poor acquisition of immunity in chronic infections. Here, we show that the high expression of FcγRIIB restricts atypical MBC responses to membrane-associated antigens that function to actively exclude FcγRIIB from the B cell immune synapse and include the co-receptor CD19, allowing B cell antigen receptor signaling and differentiation toward plasma cells. Thus, chronic infectious diseases result in the expansion of B cells that robustly respond to antigens that associate with cell surfaces, such as antigens in immune complexes, but are unable to respond to fully soluble antigens, such as self-antigens.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures

References
-
- World Health Organization, World Malaria Report 2018 (World Health Organization, Geneva, Switzerland, 2018), 165 pp.
-
- Langhorne J., Ndungu F. M., Sponaas A.-M., Marsh K., Immunity to malaria: More questions than answers. Nat. Immunol. 9, 725–732 (2008). - PubMed
-
- Weiss G. E., Traore B., Kayentao K., Ongoiba A., Doumbo S., Doumtabe D., Kone Y., Dia S., Guindo A., Traore A., Huang C.-Y., Miura K., Mircetic M., Li S., Baughman A., Narum D. L., Miller L. H., Doumbo O. K., Pierce S. K., Crompton P. D., The Plasmodium falciparum-specific human memory B cell compartment expands gradually with repeated malaria infections. PLOS Pathog. 6, e1000912 (2010). - PMC - PubMed
-
- Tran T. M., Li S., Doumbo S., Doumtabe D., Huang C. Y., Dia S., Bathily A., Sangala J., Kone Y., Traore A., Niangaly M., Dara C., Kayentao K., Ongoiba A., Doumbo O. K., Traore B., Crompton P. D., An intensive longitudinal cohort study of malian children and adults reveals no evidence of acquired immunity to Plasmodium falciparum infection. Clin. Infect. Dis. 57, 40–47 (2013). - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

