Congenital myasthenic syndrome caused by a frameshift insertion mutation in GFPT1
- PMID: 32754643
- PMCID: PMC7357421
- DOI: 10.1212/NXG.0000000000000468
Congenital myasthenic syndrome caused by a frameshift insertion mutation in GFPT1
Abstract
Objective: Description of a new variant of the glutamine-fructose-6-phosphate transaminase 1 (GFPT1) gene causing congenital myasthenic syndrome (CMS) in 3 children from 2 unrelated families.
Methods: Muscle biopsies, EMG, and whole-exome sequencing were performed.
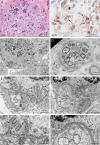
Results: All 3 patients presented with congenital hypotonia, muscle weakness, respiratory insufficiency, head lag, areflexia, and gastrointestinal dysfunction. Genetic analysis identified a homozygous frameshift insertion in the GFPT1 gene (NM_001244710.1: c.686dupC; p.Arg230Ter) that was shared by all 3 patients. In one of the patients, inheritance of the variant was through uniparental disomy (UPD) with maternal origin. Repetitive nerve stimulation and single-fiber EMG was consistent with the clinical diagnosis of CMS with a postjunctional defect. Ultrastructural evaluation of the muscle biopsy from one of the patients showed extremely attenuated postsynaptic folds at neuromuscular junctions and extensive autophagic vacuolar pathology.
Conclusions: These results expand on the spectrum of known loss-of-function GFPT1 mutations in CMS12 and in one family demonstrate a novel mode of inheritance due to UPD.
Copyright © 2020 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures


References
-
- de Souza PVS, Batistella GN de R, Lino VC, de Rezende Pinto WBV, Annes M, Oliveira ASB. Clinical and genetic basis of congenital myasthenic syndromes. Arq Neuro-Psiquiatr 2016;74:750–760. - PubMed
-
- Guergueltcheva V, Müller JS, Dusl M, et al. Congenital myasthenic syndrome with tubular aggregates caused by GFPT1 mutations. J Neurol 2011;259:838–850. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
