Real-World Clinical Practice Use of 8-Week Glecaprevir/Pibrentasvir in Treatment-Naïve Patients with Compensated Cirrhosis
- PMID: 32754824
- PMCID: PMC7444399
- DOI: 10.1007/s12325-020-01449-0
Real-World Clinical Practice Use of 8-Week Glecaprevir/Pibrentasvir in Treatment-Naïve Patients with Compensated Cirrhosis
Erratum in
-
Correction to: Real-World Clinical Practice Use of 8-Week Glecaprevir/Pibrentasvir in Treatment-Naïve Patients with Compensated Cirrhosis.Adv Ther. 2020 Nov;37(11):4755-4756. doi: 10.1007/s12325-020-01482-z. Adv Ther. 2020. PMID: 32915409 Free PMC article.
Abstract
Introduction: More than 70 million people are estimated to be infected with hepatitis C virus globally. Glecaprevir/pibrentasvir is a widely used treatment and has recently been approved for an 8-week regimen for treatment-naïve patients with compensated cirrhosis in Europe and the USA, who would previously have received glecaprevir/pibrentasvir for 12 weeks. This label update was based on the EXPEDITION-8 study, which included 343 treatment-naïve patients with compensated cirrhosis. However, there is currently a lack of similarly large-scale real-world studies of the 8-week glecaprevir/pibrentasvir regimen in this population.
Methods: This summary of seven separate smaller real-world studies aims to validate the results seen in EXPEDITION-8 and provide an up-to-date real-world reference for clinicians making treatment decisions for patients with compensated cirrhosis (Child-Pugh A) who may benefit from a shorter-duration therapy with glecaprevir/pibrentasvir. The newly emerging real-world effectiveness data on treatment-naïve patients with compensated cirrhosis treated with 8 weeks of glecaprevir/pibrentasvir help to understand where further research is needed to support patients with hepatitis C virus.
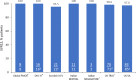
Results: Across all seven studies, glecaprevir/pibrentasvir showed high effectiveness with an average sustained virologic response rate of 98.1%, similar to that found in a clinical trial setting (99.7%). Only one patient (0.5%) experienced virologic failure and treatment was well tolerated.
Conclusion: Expanding the number of patients eligible for the shortened treatment duration will potentially increase treatment initiation and completion, particularly in underserved populations, contributing to the elimination of hepatitis C virus.
Keywords: Fibrosis; Hepatitis C; Infectious disease; Review; Therapeutics.
Figures
References
-
- World Health Organization (WHO). Hepatitis C key facts. 2019. https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-c. Accessed Mar 2020.
-
- World Health Organization (WHO). Progress report on access to hepatitis C treatment. 2018. https://apps.who.int/iris/bitstream/handle/10665/260445/WHO-CDS-HIV-18.4.... Accessed Mar 2020.
-
- World Health Organization (WHO). Global health sector strategy on viral hepatitis, 2016–2021. 2016. https://apps.who.int/iris/bitstream/handle/10665/246177/WHO-HIV-2016.06-.... Accessed Mar 2020.

