Systematic Review and Meta-Analysis of Community- and Choice-Based Health State Utility Values for Lung Cancer
- PMID: 32754857
- PMCID: PMC7547043
- DOI: 10.1007/s40273-020-00947-x
Systematic Review and Meta-Analysis of Community- and Choice-Based Health State Utility Values for Lung Cancer
Abstract
Background: Using appropriate health state utility values (HSUVs) is critical for economic evaluation of new lung cancer interventions, such as low-dose computed tomography screening and immunotherapy. Therefore, we provide a systematic review and meta-analysis of community- and choice-based HSUVs for lung cancer.
Methods: On 6 March 2017, we conducted a systematic search of the following databases: Embase, Ovid MEDLINE, Web of Science, Cochrane CENTRAL, Google Scholar, and the School of Health and Related Research Health Utility Database. The search was updated on 17 April 2019. Studies reporting mean or median lung cancer-specific HSUVs including a measure of variance were included and assessed for relevance and validity. Studies with high relevance (i.e. community- and choice-based) were further analysed. Mean HSUVs were pooled using random-effects models for all stages, stages I-II, and stages III-IV. For studies with a control group, we calculated the disutility due to lung cancer. A sensitivity analysis included only the methodologically most comparable studies (i.e. using the EQ-5D instrument and matching tariff). Subgroup analyses were conducted by time to death, histology, sex, age, treatment modality, treatment line, and progression status.
Results: We identified and analysed 27 studies of high relevance. The pooled HSUV was 0.68 (95% confidence interval [CI] 0.61-0.75) for all stages, 0.78 (95% CI 0.70-0.86) for stages I-II, and 0.69 (95% CI 0.65-0.73) for stages III-IV (p = 0.02 vs. stage I-II). Heterogeneity was present in each pooled analysis (p < 0.01; I2 = 92-99%). Disutility due to lung cancer ranged from 0.11 (95% CI 0.05-0.17) to 0.27 (95% CI 0.18-0.36). In the sensitivity analysis with the methodologically most comparable studies, stage-specific HSUVs varied by country. Such studies were only identified for Canada, China, Spain, the UK, the USA, Denmark, Germany, and Thailand. In the subgroup analysis by time to death, HSUVs for metastatic non-small-cell lung cancer ranged from 0.83 (95% CI 0.82-0.85) at ≥ 360 days from death to 0.56 (95% CI 0.46-0.66) at < 30 days from death. Among patients with metastatic non-small-cell lung cancer, HSUVs were lower for those receiving third- or fourth-line treatment and for those with progressed disease. Results of subgroup analyses by histology, sex, age, and treatment modality were ambiguous.
Conclusions: The presented evidence supports the use of stage- and country-specific HSUVs. However, such HSUVs are unavailable for most countries. Therefore, our pooled HSUVs may provide the best available stage-specific HSUVs for most countries. For metastatic non-small-cell lung cancer, adjusting for the decreased HSUVs in the last year of life may be considered, as may further stratification of HSUVs by treatment line or progression status. If required, HSUVs for other health states may be identified using our comprehensive breakdown of study characteristics.
Conflict of interest statement
HJdK, KtH, and EFB are members of the Cancer Intervention and Surveillance Modeling Network (CISNET) Lung working group (grant 1U01CA199284-01 from the National Cancer Institute). HJdK is the principal investigator of the NELSON trial (Dutch-Belgian Lung Cancer Screening Trial; Nederlands-Leuvens Longkanker Screenings onderzoek) and has received speaker fees for a lung symposium at the University of Zurich/MSD. KtH and EFB are researchers affiliated with the NELSON trial. HJdK and KtH received a grant from the University of Zurich to assess the cost effectiveness of computer tomography (CT) lung cancer screening in Switzerland. HJdK and KtH were involved in the Cancer Care Ontario Health Technology Assessment Study for CT Lung Cancer Screening in Canada. KtH is involved in the SELECT (Selection of Eligible People for Lung Cancer Screening using Electronic Primary Care DaTa) study. KtH was an invited speaker at the 17th, 19th, and 20th World Conferences on Lung Cancer and the 5th Russian Society of Clinical Oncology conference, for which travel expenses were paid (in part).
Figures



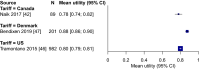


Similar articles
-
Health State Utility Values of Type 2 Diabetes Mellitus and Related Complications: A Systematic Review and Meta-Analysis.Value Health Reg Issues. 2023 Mar;34:14-22. doi: 10.1016/j.vhri.2022.09.005. Epub 2022 Nov 11. Value Health Reg Issues. 2023. PMID: 36371899
-
Health state utility values by cancer stage: a systematic literature review.Eur J Health Econ. 2021 Nov;22(8):1275-1288. doi: 10.1007/s10198-021-01335-8. Epub 2021 Jun 14. Eur J Health Econ. 2021. PMID: 34125315 Free PMC article.
-
Health state utility values in major depressive disorder treated with pharmacological interventions: a systematic literature review.Health Qual Life Outcomes. 2021 Mar 18;19(1):94. doi: 10.1186/s12955-021-01723-x. Health Qual Life Outcomes. 2021. PMID: 33736649 Free PMC article.
-
Systematic Review and Meta-Analysis of Health State Utility Values for Osteoarthritis-Related Conditions.Arthritis Care Res (Hoboken). 2022 Feb;74(2):291-300. doi: 10.1002/acr.24478. Epub 2022 Jan 11. Arthritis Care Res (Hoboken). 2022. PMID: 33026702
-
Health state utility values in patients with Ankylosing Spondylitis: a systematic review and meta-analysis.Qual Life Res. 2024 Sep;33(9):2321-2334. doi: 10.1007/s11136-024-03670-8. Epub 2024 Jun 1. Qual Life Res. 2024. PMID: 38824212
Cited by
-
Cost-effectiveness of lung cancer screening with volume computed tomography in Portugal.J Comp Eff Res. 2024 Nov;13(11):e240102. doi: 10.57264/cer-2024-0102. Epub 2024 Sep 27. J Comp Eff Res. 2024. PMID: 39329332 Free PMC article.
-
Health State Utility Values in Early-Stage Non-small Cell Lung Cancer: A Systematic Literature Review.Pharmacoecon Open. 2023 Sep;7(5):723-738. doi: 10.1007/s41669-023-00423-0. Epub 2023 Jun 8. Pharmacoecon Open. 2023. PMID: 37289325 Free PMC article.
-
Association between out-of-pocket expenditure and health-related quality of life among patients receiving cancer treatment: a cross-sectional study from Nepal.Health Qual Life Outcomes. 2025 Jul 15;23(1):73. doi: 10.1186/s12955-025-02404-9. Health Qual Life Outcomes. 2025. PMID: 40660255 Free PMC article.
-
The Cost-Effectiveness of Lorlatinib Versus Chemotherapy as a Second- or Third-Line Treatment in Anaplastic Lymphoma Kinase (ALK)-Positive Non-small-cell Lung Cancer in Sweden.Pharmacoeconomics. 2021 Aug;39(8):941-952. doi: 10.1007/s40273-021-01015-8. Epub 2021 Jun 3. Pharmacoeconomics. 2021. PMID: 34080140 Free PMC article. Clinical Trial.
-
Effects of medical interventions on health-related quality of life in chronic disease - systematic review and meta-analysis of the 19 most common diagnoses.Front Public Health. 2024 Feb 6;12:1313685. doi: 10.3389/fpubh.2024.1313685. eCollection 2024. Front Public Health. 2024. PMID: 38379671 Free PMC article.
References
-
- International Agency for Research on Cancer. Cancer Today (powered by GLOBOCAN 2018). https://publications.iarc.fr/Databases/Iarc-Cancerbases/Cancer-Today-Pow.... Accessed 11 Nov 2019.
-
- Neumann PJ, Goldie SJ, Weinstein MC. Preference-based measures in economic evaluation in health care. Annu Rev Public Health. 2000;21:587–611. - PubMed
-
- National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013. nice.org.uk/process/pmg9. Published: 4 Apr 2013; Accessed 26 Nov 2019. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

