Multi-parametric MR in Becker muscular dystrophy patients
- PMID: 32754921
- PMCID: PMC7687231
- DOI: 10.1002/nbm.4385
Multi-parametric MR in Becker muscular dystrophy patients
Abstract
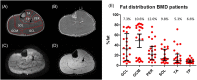
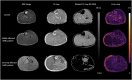
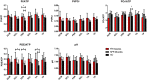
Quantitative MRI and MRS of muscle are increasingly being used to measure individual pathophysiological processes in Becker muscular dystrophy (BMD). In particular, muscle fat fraction was shown to be highly associated with functional tests in BMD. However, the muscle strength per unit of contractile cross-sectional area is lower in patients with BMD compared with healthy controls. This suggests that the quality of the non-fat-replaced (NFR) muscle tissue is lower than in healthy controls. Consequently, a measure that reflects changes in muscle tissue itself is needed. Here, we explore the potential of water T2 relaxation times, diffusion parameters and phosphorus metabolic indices as early disease markers in patients with BMD. For this purpose, we examined these measures in fat-replaced (FR) and NFR lower leg muscles in patients with BMD and compared these values with those in healthy controls. Quantitative proton MRI (three-point Dixon, multi-spin-echo and diffusion-weighted spin-echo echo planar imaging) and 2D chemical shift imaging 31 P MRS data were acquired in 24 patients with BMD (age 18.8-66.2 years) and 13 healthy controls (age 21.3-63.6 years). Muscle fat fractions, phosphorus metabolic indices, and averages and standard deviations (SDs) of the water T2 relaxation times and diffusion tensor imaging (DTI) parameters were assessed in six individual leg muscles. Phosphodiester levels were increased in the NFR and FR tibialis anterior, FR peroneus and FR gastrocnemius lateralis muscles. No clear pattern was visible for the other metabolic indices. Increased T2 SD was found in the majority of FR muscles compared with NFR and healthy control muscles. No differences in average water T2 relaxation times or DTI indices were found between groups. Overall, our results indicate that primarily muscles that are further along in the disease process showed increases in T2 heterogeneity and changes in some metabolic indices. No clear differences were found for the DTI indices between groups.
Keywords: Becker muscular dystrophy; phosphorus MRS; quantitative MRI; skeletal muscle.
© 2020 The Authors. NMR in Biomedicine published by John Wiley & Sons Ltd.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

