Phase II Randomized Trial of Rituximab Plus Cyclophosphamide Followed by Belimumab for the Treatment of Lupus Nephritis
- PMID: 32755035
- PMCID: PMC7839443
- DOI: 10.1002/art.41466
Phase II Randomized Trial of Rituximab Plus Cyclophosphamide Followed by Belimumab for the Treatment of Lupus Nephritis
Erratum in
-
Incorrect Frequency of Belimumab Infusions Reported in the Article by Atisha-Fregoso et al (Arthritis Rheumatol, January 2021).Arthritis Rheumatol. 2021 Dec;73(12):2356. doi: 10.1002/art.42019. Arthritis Rheumatol. 2021. PMID: 34890126 Free PMC article. No abstract available.
Abstract
Objective: To assess the safety, mechanism of action, and preliminary efficacy of rituximab followed by belimumab in the treatment of refractory lupus nephritis (LN).
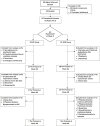
Methods: In a multicenter, randomized, open-label clinical trial, 43 patients with recurrent or refractory LN were treated with rituximab, cyclophosphamide (CYC), and glucocorticoids followed by weekly belimumab infusions until week 48 (RCB group), or treated with rituximab and CYC but no belimumab infusions (RC group). Patients were followed up until week 96. Percentages of total and autoreactive B cell subsets in the patients' peripheral blood were analyzed by flow cytometry.
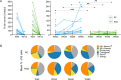
Results: Treatment with belimumab did not increase the incidence of adverse events in patients with refractory LN. At week 48, a complete or partial renal response occurred in 11 (52%) of 21 patients receiving belimumab, compared to 9 (41%) of 22 patients in the RC group who did not receive belimumab (P = 0.452). Lack of improvement in or worsening of LN was the major reason for treatment failure. B cell depletion occurred in both groups, but the percentage of B cells remained lower in those receiving belimumab (geometric mean number of B cells at week 60, 53 cells/μl in the RCB group versus 11 cells/μl in the RC group; P = 0.0012). Percentages of total and autoreactive transitional B cells increased from baseline to week 48 in both groups. However, percentages of total and autoreactive naive B cells decreased from baseline to week 48 in the belimumab group compared to the no belimumab RC group (P = 0.0349), a finding that is consistent with the observed impaired maturation of naive B cells and enhanced censoring of autoreactive B cells.
Conclusion: The addition of belimumab to a treatment regimen with rituximab and CYC was safe in patients with refractory LN. This regimen diminished maturation of transitional to naive B cells during B cell reconstitution, and enhanced the negative selection of autoreactive B cells. Clinical efficacy was not improved with rituximab and CYC in combination with belimumab when compared to a therapeutic strategy of B cell depletion alone in patients with LN.
Trial registration: ClinicalTrials.gov NCT02260934.
© 2020 The Authors. Arthritis & Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures

References
-
- Mok CC, Kwok RC, Yip PS. Effect of renal disease on the standardized mortality ratio and life expectancy of patients with systemic lupus erythematosus. Arthritis Rheum 2013;65:2154–60. - PubMed
-
- Dall'Era M. Treatment of lupus nephritis: current paradigms and emerging strategies [review]. Curr Opin Rheumatol 2017;29:241–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

