Addressing the quality of submissions to ClinicalTrials.gov for registration and results posting: The use of a checklist
- PMID: 32755266
- PMCID: PMC7655525
- DOI: 10.1177/1740774520942746
Addressing the quality of submissions to ClinicalTrials.gov for registration and results posting: The use of a checklist
Abstract
Background: US Federal regulations since the late 1990s have required registration of some clinical trials and submission of results for some of these trials on a public registry, ClinicalTrials.gov. The quality of the submissions made to ClinicalTrials.gov determines the duration of the Quality Control review, whether the submission will pass the review (success), and how many review cycles it will take for a study to be posted. Success rate for all results submitted to ClinicalTrials.gov is less than 25%. To increase the success of investigators' submissions and meet the requirements of registration and submission of results in a timely fashion, the Johns Hopkins ClinicalTrials.gov Program implemented a policy to review all studies for quality before submission. To standardize our review for quality, minimize inter-reviewer variability, and have a tool for training new staff, we developed a checklist.
Methods: The Program staff learned from major comments received from ClinicalTrials.gov and also reviewed the Protocol Registration and Results System review criteria for registration and results to fully understand how to prepare studies to pass Quality Control review. These were summarized into bulleted points and incorporated into a checklist used by Program staff to review studies before submission.
Results: In the period before the introduction of the checklist, 107 studies were submitted for registration with a 45% (48/107) success rate, a mean (SD) of 18.9 (26.72) days in review, and 1.74 (0.78) submission cycles. Results for 44 records were submitted with 11% (5/44) success rate, 115.80 (129.33) days in review, and 2.23 (0.68) submission cycles. In the period after the checklist, 104 studies were submitted for registration with 80% (83/104) success rate, 2.12 (3.85) days in review, and 1.22 (0.46) submission cycles. Results for 22 records were submitted with 41% (9/22) success rate, 39.27 (19.84) days in review, and 1.64 (0.58) submission cycles. Of the 44 results submitted prior to the checklist, 30 were Applicable or Probable Applicable Clinical Trials, with 10% (3/30) being posted within 30 days as required of the National Institutes of Health. For the 22 results submitted after the checklist, 17 were Applicable or Probable Applicable Clinical Trials, with 47% (8/17) being posted within 30 days of submission. These pre- and post-checklist differences were statistically significant improvements.
Conclusion: The checklist has substantially improved our success rate and contributed to a reduction in the review days and number of review cycles. If Academic Medical Centers and industry will adopt or create a similar checklist to review their studies before submission, the quality of the submissions can be improved and the duration of review can be minimized.
Keywords: Checklist; ClinicalTrials.gov; clinical trials; quality; registration; results; success rate.
Conflict of interest statement
Conflict of Interest
Authors have no conflict of interest to declare
Figures
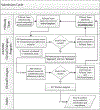

References
-
- Food and Drug Administration Modernization Act of 1997. Public Law 105–115. https://www.govinfo.gov/content/pkg/PLAW-105publ115/pdf/PLAW-105publ115.... (1997, accessed 18 November 2019).
-
- Hwang TJ, Carpenter D, Lauffenburger JC, et al. Failure of Investigational Drugs in Late-Stage Clinical Development and Publication of Trial Results. JAMA Intern Med 2016; 176(12):1826–1833. - PubMed
-
- De Angelis C, Drazen JM, Frizelle FA, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. N Engl J Med 2004; 351:1250–1251. - PubMed
-
- Food and Drug Administration Amendments Act of 2007. Public Law 110–85. https://www.govinfo.gov/content/pkg/PLAW-110publ85/pdf/PLAW-110publ85.pd... (2007, accessed 18 November 2019).
-
- Zarin DA, Fain KM, Dobbins HD, et al. 10-Year Update on Study Results Submitted to ClinicalTrials.gov. N Engl J Med 2019; 381:1966–1974. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

