Acetaldehyde suppresses HBV-MHC class I complex presentation on hepatocytes via induction of ER stress and Golgi fragmentation
- PMID: 32755306
- PMCID: PMC7654643
- DOI: 10.1152/ajpgi.00109.2020
Acetaldehyde suppresses HBV-MHC class I complex presentation on hepatocytes via induction of ER stress and Golgi fragmentation
Abstract
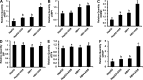
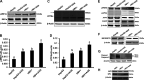
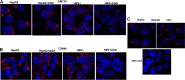
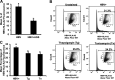
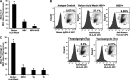
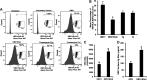
Alcohol consumption worsens hepatitis B virus (HBV) infection pathogenesis. We have recently reported that acetaldehyde suppressed HBV peptide-major histocompatibility complex I (MHC class I) complex display on hepatocytes, limiting recognition and subsequent removal of the infected hepatocytes by HBV-specific cytotoxic T lymphocytes (CTLs). This suppression was attributed to impaired processing of antigenic peptides by the proteasome. However, in addition to proteasome dysfunction, alcohol may induce endoplasmic reticulum (ER) stress and Golgi fragmentation in HBV-infected liver cells to reduce uploading of viral peptides to MHC class I and/or trafficking of this complex to the hepatocyte surface. Hence, the aim of this study was to elucidate whether alcohol-induced ER stress and Golgi fragmentation affect HBV peptide-MHC class I complex presentation on HBV+ hepatocytes. Here, we demonstrate that, while both acetaldehyde and HBV independently cause ER stress and Golgi fragmentation, the combined exposure provided an additive effect. Thus we observed an activation of the inositol-requiring enzyme 1α-X-box binding protein 1 and activation transcription factor (ATF)6α, but not the phospho PKR-like ER kinase-phospho eukaryotic initiation factor 2α-ATF4-C/EBP homologous protein arms of ER stress in HBV-transfected cells treated with acetaldehyde-generating system (AGS). In addition, Golgi proteins trans-Golgi network 46, GM130, and Giantin revealed punctate distribution, indicating Golgi fragmentation upon AGS exposure. Furthermore, the effects of acetaldehyde were reproduced by treatment with ER stress inducers, thapsigargin and tunicamycin, which also decreased the display of this complex and MHC class I turnover in HepG2.2.15 cells and HBV-infected primary human hepatocytes. Taken together, alcohol-induced ER stress and Golgi fragmentation contribute to the suppression of HBV peptide-MHC class I complex presentation on HBV+ hepatocytes, which may diminish their recognition by CTLs and promote persistence of HBV infection in hepatocytes.NEW & NOTEWORTHY Our current findings show that acetaldehyde accelerates endoplasmic reticulum (ER) stress by activating the unfolded protein response arms inositol-requiring enzyme 1α-X-box binding protein 1 and activation transcription factor (ATF)6α but not phospho PKR-like ER kinase-p eukaryotic initiation factor 2α-ATF4-C/EBP homologous protein in hepatitis B virus (HBV)-transfected HepG2.2.15 cells. It also potentiates Golgi fragmentation, as evident by punctate distribution of Golgi proteins, GM130, trans-Golgi network 46, and Giantin. While concomitantly increasing HBV DNA and HBV surface antigen titers, acetaldehyde-induced ER stress suppresses the presentation of HBV peptide-major histocompatibility complex I complexes on hepatocyte surfaces, thereby promoting the persistence of HBV infection in the liver.
Keywords: ER stress; Golgi; HBV; acetaldehyde; hepatocytes.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


Similar articles
-
Ethanol attenuates presentation of cytotoxic T-lymphocyte epitopes on hepatocytes of HBV-infected humanized mice.Alcohol Clin Exp Res. 2022 Jan;46(1):40-51. doi: 10.1111/acer.14740. Epub 2021 Nov 23. Alcohol Clin Exp Res. 2022. PMID: 34773268 Free PMC article.
-
Acetaldehyde suppresses the display of HBV-MHC class I complexes on HBV-expressing hepatocytes.Am J Physiol Gastrointest Liver Physiol. 2019 Aug 1;317(2):G127-G140. doi: 10.1152/ajpgi.00064.2019. Epub 2019 May 29. Am J Physiol Gastrointest Liver Physiol. 2019. PMID: 31141391 Free PMC article.
-
Apolipoprotein H drives hepatitis B surface antigen retention and endoplasmic reticulum stress during hepatitis B virus infection.Int J Biochem Cell Biol. 2021 Feb;131:105906. doi: 10.1016/j.biocel.2020.105906. Epub 2020 Dec 26. Int J Biochem Cell Biol. 2021. PMID: 33370716
-
Naturally Occurring Hepatitis B Virus Mutations Leading to Endoplasmic Reticulum Stress and Their Contribution to the Progression of Hepatocellular Carcinoma.Int J Mol Sci. 2019 Jan 30;20(3):597. doi: 10.3390/ijms20030597. Int J Mol Sci. 2019. PMID: 30704071 Free PMC article. Review.
-
ER stress-induced cell death mechanisms.Biochim Biophys Acta. 2013 Dec;1833(12):3460-3470. doi: 10.1016/j.bbamcr.2013.06.028. Epub 2013 Jul 10. Biochim Biophys Acta. 2013. PMID: 23850759 Free PMC article. Review.
Cited by
-
The role of the Golgi apparatus in disease (Review).Int J Mol Med. 2021 Apr;47(4):38. doi: 10.3892/ijmm.2021.4871. Epub 2021 Feb 4. Int J Mol Med. 2021. PMID: 33537825 Free PMC article. Review.
-
Molecular Changes in Relation to Alcohol Consumption and Hepatocellular Carcinoma.Int J Mol Sci. 2022 Aug 26;23(17):9679. doi: 10.3390/ijms23179679. Int J Mol Sci. 2022. PMID: 36077080 Free PMC article. Review.
-
Endoplasmic Reticulum Stress in Hepatitis B Virus and Hepatitis C Virus Infection.Viruses. 2022 Nov 25;14(12):2630. doi: 10.3390/v14122630. Viruses. 2022. PMID: 36560634 Free PMC article. Review.
-
Hepatic progesterone receptor membrane component 1 attenuates ethanol-induced liver injury by reducing acetaldehyde production and oxidative stress.Am J Physiol Gastrointest Liver Physiol. 2023 Jun 1;324(6):G442-G451. doi: 10.1152/ajpgi.00206.2022. Epub 2023 Apr 18. Am J Physiol Gastrointest Liver Physiol. 2023. PMID: 37070746 Free PMC article.
-
Oncolytic Virus-Induced Autophagy in Glioblastoma.Cancers (Basel). 2021 Jul 12;13(14):3482. doi: 10.3390/cancers13143482. Cancers (Basel). 2021. PMID: 34298694 Free PMC article. Review.
References
-
- Bertoletti A, Costanzo A, Chisari FV, Levrero M, Artini M, Sette A, Penna A, Giuberti T, Fiaccadori F, Ferrari C. Cytotoxic T lymphocyte response to a wild type hepatitis B virus epitope in patients chronically infected by variant viruses carrying substitutions within the epitope. J Exp Med 180: 933–943, 1994. doi:10.1084/jem.180.3.933. - DOI - PMC - PubMed
-
- Bertoletti A, Southwood S, Chesnut R, Sette A, Falco M, Ferrara GB, Penna A, Boni C, Fiaccadori F, Ferrari C. Molecular features of the hepatitis B virus nucleocapsid T-cell epitope 18–27: interaction with HLA and T-cell receptor. Hepatology 26: 1027–1034, 1997. doi:10.1002/hep.510260435. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

