Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated With Respiratory Failure and Coagulopathy
- PMID: 32755393
- PMCID: PMC7497892
- DOI: 10.1161/CIRCULATIONAHA.120.048488
Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated With Respiratory Failure and Coagulopathy
Abstract
Background: Severe acute respiratory syndrome corona virus 2 infection causes severe pneumonia (coronavirus disease 2019 [COVID-19]), but the mechanisms of subsequent respiratory failure and complicating renal and myocardial involvement are poorly understood. In addition, a systemic prothrombotic phenotype has been reported in patients with COVID-19.
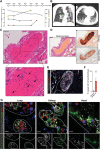
Methods: A total of 62 subjects were included in our study (n=38 patients with reverse transcriptase polymerase chain reaction-confirmed COVID-19 and n=24 non-COVID-19 controls). We performed histopathologic assessment of autopsy cases, surface marker-based phenotyping of neutrophils and platelets, and functional assays for platelet, neutrophil functions, and coagulation tests, as well.
Results: We provide evidence that organ involvement and prothrombotic features in COVID-19 are linked by immunothrombosis. We show that, in COVID-19, inflammatory microvascular thrombi are present in the lung, kidney, and heart, containing neutrophil extracellular traps associated with platelets and fibrin. Patients with COVID-19 also present with neutrophil-platelet aggregates and a distinct neutrophil and platelet activation pattern in blood, which changes with disease severity. Whereas cases of intermediate severity show an exhausted platelet and hyporeactive neutrophil phenotype, patients severely affected with COVID-19 are characterized by excessive platelet and neutrophil activation in comparison with healthy controls and non-COVID-19 pneumonia. Dysregulated immunothrombosis in severe acute respiratory syndrome corona virus 2 pneumonia is linked to both acute respiratory distress syndrome and systemic hypercoagulability.
Conclusions: Taken together, our data point to immunothrombotic dysregulation as a key marker of disease severity in COVID-19. Further work is necessary to determine the role of immunothrombosis in COVID-19.
Keywords: COVID-19; blood platelets; disseminated intravascular coagulation; neutrophils; respiratory insufficiency; severe acute respiratory syndrome coronavirus 2; thrombosis.
Conflict of interest statement
None.
Figures



References
-
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

