Human Cardiac Mesenchymal Stem Cells Remodel in Disease and Can Regulate Arrhythmia Substrates
- PMID: 32755466
- PMCID: PMC7578059
- DOI: 10.1161/CIRCEP.120.008740
Human Cardiac Mesenchymal Stem Cells Remodel in Disease and Can Regulate Arrhythmia Substrates
Abstract
Background: The mesenchymal stem cell (MSC), known to remodel in disease and have an extensive secretome, has recently been isolated from the human heart. However, the effects of normal and diseased cardiac MSCs on myocyte electrophysiology remain unclear. We hypothesize that in disease the inflammatory secretome of cardiac human MSCs (hMSCs) remodels and can regulate arrhythmia substrates.
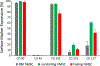
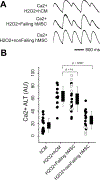
Methods: hMSCs were isolated from patients with or without heart failure from tissue attached to extracted device leads and from samples taken from explanted/donor hearts. Failing hMSCs or nonfailing hMSCs were cocultured with normal human cardiac myocytes derived from induced pluripotent stem cells. Using fluorescent indicators, action potential duration, Ca2+ alternans, and spontaneous calcium release (SCR) incidence were determined.
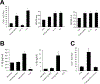
Results: Failing and nonfailing hMSCs from both sources exhibited similar trilineage differentiation potential and cell surface marker expression as bone marrow hMSCs. Compared with nonfailing hMSCs, failing hMSCs prolonged action potential duration by 24% (P<0.001, n=15), increased Ca2+ alternans by 300% (P<0.001, n=18), and promoted spontaneous calcium release activity (n=14, P<0.013) in human cardiac myocytes derived from induced pluripotent stem cells. Failing hMSCs exhibited increased secretion of inflammatory cytokines IL (interleukin)-1β (98%, P<0.0001) and IL-6 (460%, P<0.02) compared with nonfailing hMSCs. IL-1β or IL-6 in the absence of hMSCs prolonged action potential duration but only IL-6 increased Ca2+ alternans and promoted spontaneous calcium release activity in human cardiac myocytes derived from induced pluripotent stem cells, replicating the effects of failing hMSCs. In contrast, nonfailing hMSCs prevented Ca2+ alternans in human cardiac myocytes derived from induced pluripotent stem cells during oxidative stress. Finally, nonfailing hMSCs exhibited >25× higher secretion of IGF (insulin-like growth factor)-1 compared with failing hMSCs. Importantly, IGF-1 supplementation or anti-IL-6 treatment rescued the arrhythmia substrates induced by failing hMSCs.
Conclusions: We identified device leads as a novel source of cardiac hMSCs. Our findings show that cardiac hMSCs can regulate arrhythmia substrates by remodeling their secretome in disease. Importantly, therapy inhibiting (anti-IL-6) or mimicking (IGF-1) the cardiac hMSC secretome can rescue arrhythmia substrates.
Keywords: COVID-19; arrhythmia; insulin-like growth factor 1; interleukin-6; mesenchymal stem cell.
Figures





Similar articles
-
Mesenchymal stem cells stimulate protective genetic reprogramming of injured cardiac ventricular myocytes.J Mol Cell Cardiol. 2011 Feb;50(2):346-56. doi: 10.1016/j.yjmcc.2010.09.001. Epub 2010 Sep 15. J Mol Cell Cardiol. 2011. PMID: 20837021
-
Mesenchymal stem cells suppress cardiac alternans by activation of PI3K mediated nitroso-redox pathway.J Mol Cell Cardiol. 2016 Sep;98:138-45. doi: 10.1016/j.yjmcc.2016.05.014. Epub 2016 May 26. J Mol Cell Cardiol. 2016. PMID: 27238412 Free PMC article.
-
Calcium entry via Na/Ca exchange during the action potential directly contributes to contraction of failing human ventricular myocytes.Cardiovasc Res. 2003 Mar 15;57(4):974-85. doi: 10.1016/s0008-6363(02)00732-0. Cardiovasc Res. 2003. PMID: 12650875
-
Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes as Models for Cardiac Channelopathies: A Primer for Non-Electrophysiologists.Circ Res. 2018 Jul 6;123(2):224-243. doi: 10.1161/CIRCRESAHA.118.311209. Circ Res. 2018. PMID: 29976690 Free PMC article. Review.
-
The origin and arrhythmogenic potential of fibroblasts in cardiac disease.J Cardiovasc Transl Res. 2012 Dec;5(6):760-7. doi: 10.1007/s12265-012-9408-1. Epub 2012 Sep 18. J Cardiovasc Transl Res. 2012. PMID: 22987310 Free PMC article. Review.
Cited by
-
Interleukin-6 Elevation Is a Key Pathogenic Factor Underlying COVID-19-Associated Heart Rate-Corrected QT Interval Prolongation.Front Cardiovasc Med. 2022 May 19;9:893681. doi: 10.3389/fcvm.2022.893681. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35665254 Free PMC article.
-
Fir(e)ing the Rhythm: Inflammatory Cytokines and Cardiac Arrhythmias.JACC Basic Transl Sci. 2023 Feb 15;8(6):728-750. doi: 10.1016/j.jacbts.2022.12.004. eCollection 2023 Jun. JACC Basic Transl Sci. 2023. PMID: 37426535 Free PMC article. Review.
-
Shenfu injection: a review of pharmacological effects on cardiovascular diseases.Front Pharmacol. 2024 Feb 14;15:1279584. doi: 10.3389/fphar.2024.1279584. eCollection 2024. Front Pharmacol. 2024. PMID: 38420190 Free PMC article. Review.
-
Arrhythmias including atrial fibrillation and congenital heart disease in Kleefstra syndrome: a possible epigenetic link.Europace. 2023 Dec 28;26(1):euae003. doi: 10.1093/europace/euae003. Europace. 2023. PMID: 38195854 Free PMC article.
-
Integrated Analysis of the microRNA-mRNA Network Predicts Potential Regulators of Atrial Fibrillation in Humans.Cells. 2022 Aug 24;11(17):2629. doi: 10.3390/cells11172629. Cells. 2022. PMID: 36078037 Free PMC article.
References
-
- Wagner W, Roderburg C, Wein F, Diehlmann A, Frankhauser M, Schubert R, Eckstein V, Ho AD. Molecular and secretory profiles of human mesenchymal stromal cells and their abilities to maintain primitive hematopoietic progenitors. Stem Cells. 2007;25:2638–2647. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

