Validation of a single-step, single-tube reverse transcription loop-mediated isothermal amplification assay for rapid detection of SARS-CoV-2 RNA
- PMID: 32755529
- PMCID: PMC7656183
- DOI: 10.1099/jmm.0.001238
Validation of a single-step, single-tube reverse transcription loop-mediated isothermal amplification assay for rapid detection of SARS-CoV-2 RNA
Abstract
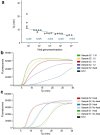
Introduction. The SARS-CoV-2 pandemic of 2020 has resulted in unparalleled requirements for RNA extraction kits and enzymes required for virus detection, leading to global shortages. This has necessitated the exploration of alternative diagnostic options to alleviate supply chain issues.Aim. To establish and validate a reverse transcription loop-mediated isothermal amplification (RT- LAMP) assay for the detection of SARS-CoV-2 from nasopharyngeal swabs.Methodology. We used a commercial RT-LAMP mastermix from OptiGene in combination with a primer set designed to detect the CDC N1 region of the SARS-CoV-2 nucleocapsid (N) gene. A single-tube, single-step fluorescence assay was implemented whereby 1 µl of universal transport medium (UTM) directly from a nasopharyngeal swab could be used as template, bypassing the requirement for RNA purification. Amplification and detection could be conducted in any thermocycler capable of holding 65 °C for 30 min and measure fluorescence in the FAM channel at 1 min intervals.Results. Assay evaluation by assessment of 157 clinical specimens previously screened by E-gene RT-qPCR revealed assay sensitivity and specificity of 87 and 100%, respectively. Results were fast, with an average time-to-positive (Tp) for 93 clinical samples of 14 min (sd±7 min). Using dilutions of SARS-CoV-2 virus spiked into UTM, we also evaluated assay performance against FDA guidelines for implementation of emergency-use diagnostics and established a limit-of-detection of 54 Tissue Culture Infectious Dose 50 per ml (TCID50 ml-1), with satisfactory assay sensitivity and specificity. A comparison of 20 clinical specimens between four laboratories showed excellent interlaboratory concordance; performing equally well on three different, commonly used thermocyclers, pointing to the robustness of the assay.Conclusion. With a simplified workflow, The N1 gene Single Tube Optigene LAMP assay (N1-STOP-LAMP) is a powerful, scalable option for specific and rapid detection of SARS-CoV-2 and an additional resource in the diagnostic armamentarium against COVID-19.
Keywords: RT-LAMP; SARS-CoV-2; nasopharyngeal swabs; universal transport media.
Conflict of interest statement
The authors declare the following conflict of interest: Nickala Best, Sean McDonald and Arran Greenhalgh are employees of GeneWorks, a commercial entity that distributes OptiGene reagents in Australia.
Figures


References
-
- Victorian Department Health and Human Services 2020 Coronavirus disease 2019 (COVID-19) Guidelines for health services and general practitioners - Version 17, 5/04/2020. State Government of Victoria, Australia. https://www.dhhs.vic.gov.au/health-services-and-general-practitioners-co... 7 April 2020.
-
- UK National Health Service Guidance and standard operating procedure: COVID-19 virus testing in NHS laboratories, 16/03/2020. NHS, London. 2020 https://www.england.nhs.uk/coronavirus/publication/guidance-and-standard... 7 April 2020.
-
- Center for Disease Control A 2019-Novel Coronavirus (2019-nCoV) real-time RT-PCR panel primers and probes, 24/01/2020. US Department of Health & Human Services, CDC, Atlanta. 2020 https://www.cdc.gov/coronavirus/2019-ncov/downloads/rt-pcr-panel-primer-... 7 April 2020.
-
- Centre for Disease Control Real-time RT-PCR panel for detection 2019-Novel Coronavirus, 24/01/2020. US Department of Health & Human Services, CDC, Atlanta. 2020 https://www.cdc.gov/coronavirus/2019-ncov/downloads/rt-pcr-panel-for-det... 7 April 2020.
-
- Food and Drug Administration. 2020 Policy for diagnostic tests for Coronavirus disease – 2019 during the public health emergency – Immediately in effect guidance for clinical laboratories, commercial manufacturers, and food and Drug administration staff, 16/03/2020. US Department of Health & Human Services, Food and Drug Administration. https://www.fda.gov/regulatory-information/search-fda-guidance-documents... 7 April 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

