Cdc13 is predominant over Stn1 and Ten1 in preventing chromosome end fusions
- PMID: 32755541
- PMCID: PMC7406354
- DOI: 10.7554/eLife.53144
Cdc13 is predominant over Stn1 and Ten1 in preventing chromosome end fusions
Abstract
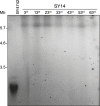
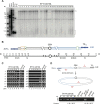
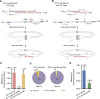
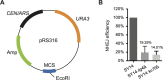
Telomeres define the natural ends of eukaryotic chromosomes and are crucial for chromosomal stability. The budding yeast Cdc13, Stn1 and Ten1 proteins form a heterotrimeric complex, and the inactivation of any of its subunits leads to a uniformly lethal phenotype due to telomere deprotection. Although Cdc13, Stn1 and Ten1 seem to belong to an epistasis group, it remains unclear whether they function differently in telomere protection. Here, we employed the single-linear-chromosome yeast SY14, and surprisingly found that the deletion of CDC13 leads to telomere erosion and intrachromosome end-to-end fusion, which depends on Rad52 but not Yku. Interestingly, the emergence frequency of survivors in the SY14 cdc13Δ mutant was ~29 fold higher than that in either the stn1Δ or ten1Δ mutant, demonstrating a predominant role of Cdc13 in inhibiting telomere fusion. Chromosomal fusion readily occurred in the telomerase-null SY14 strain, further verifying the default role of intact telomeres in inhibiting chromosome fusion.
Keywords: CST complex; S. cerevisiae; chromosome end fusion; genetics; genomics; homologous recombination; single chromosome yeast; telomere protection.
© 2020, Wu et al.
Conflict of interest statement
ZW, JL, XM, XG, TL, CC, MH, YS, NL, XX, ZQ, JZ No competing interests declared
Figures























Comment in
-
Getting to grips with circular chromosomes.Elife. 2020 Aug 5;9:e60150. doi: 10.7554/eLife.60150. Elife. 2020. PMID: 32755540 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

