Viral Vectors for Neural Circuit Mapping and Recent Advances in Trans-synaptic Anterograde Tracers
- PMID: 32755550
- PMCID: PMC7530073
- DOI: 10.1016/j.neuron.2020.07.010
Viral Vectors for Neural Circuit Mapping and Recent Advances in Trans-synaptic Anterograde Tracers
Abstract
Viral tracers are important tools for neuroanatomical mapping and genetic payload delivery. Genetically modified viruses allow for cell-type-specific targeting and overcome many limitations of non-viral tracers. Here, we summarize the viruses that have been developed for neural circuit mapping, and we provide a primer on currently applied anterograde and retrograde viral tracers with practical guidance on experimental uses. We also discuss and highlight key technical and conceptual considerations for developing new safer and more effective anterograde trans-synaptic viral vectors for neural circuit analysis in multiple species.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures
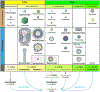


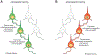

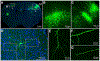

References
-
- Archin NM, and Atherton SS (2002). Rapid spread of a neurovirulent strain of HSV-1 through the CNS of BALB/c mice following anterior chamber inoculation. J Neurovirol 8, 122–135. - PubMed
-
- Archin NM, van den Boom L, Perelygina L, Hilliard JM, and Atherton SS (2003). Delayed spread and reduction in virus titer after anterior chamber inoculation of a recombinant of HSV-1 expressing IL-16. Invest Ophthalmol Vis Sci 44, 3066–3076. - PubMed
-
- Barsoum J, Brown R, McKee M, and Boyce FM (1997). Efficient transduction of mammalian cells by a recombinant baculovirus having the vesicular stomatitis virus G glycoprotein. Hum Gene Ther 8, 2011–2018. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

