Stromal Cell-Contact Dependent PI3K and APRIL Induced NF-κB Signaling Prevent Mitochondrial- and ER Stress Induced Death of Memory Plasma Cells
- PMID: 32755576
- PMCID: PMC7408492
- DOI: 10.1016/j.celrep.2020.107982
Stromal Cell-Contact Dependent PI3K and APRIL Induced NF-κB Signaling Prevent Mitochondrial- and ER Stress Induced Death of Memory Plasma Cells
Abstract
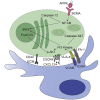
The persistence of long-lived memory plasma cells in the bone marrow depends on survival factors available in the bone marrow, which are provided in niches organized by stromal cells. Using an ex vivo system in which we supply the known survival signals, direct cell contact to stromal cells, and the soluble cytokine a proliferation-inducing ligand (APRIL), we have elucidated the critical signaling pathways required for the survival of long-lived plasma cells. Integrin-mediated contact of bone marrow plasma cells with stromal cells activates the phosphatidylinositol 3-kinase (PI3K) signaling pathway, leading to critical inactivation of Forkhead-Box-Protein O1/3 (FoxO1/3) and preventing the activation of mitochondrial stress-associated effector caspases 3 and 7. Accordingly, inhibition of PI3K signaling in vivo ablates bone marrow plasma cells. APRIL signaling, by the nuclear factor κB (NF-κB) pathway, blocks activation of the endoplasmic-reticulum-stress-associated initiator caspase 12. Thus, stromal-cell-contact-induced PI3K and APRIL-induced NF-κB signaling provide the necessary and complementary signals to maintain bone marrow memory plasma cells.
Keywords: APRIL; BCMA; FoxO; IRF4; PI3K/AKT; bone marrow; caspase 12; caspase 3; caspase 7; long-lived memory PCs; stromal cell.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interest The authors declare no competing interests.
Figures




References
-
- Belnoue E., Pihlgren M., McGaha T.L., Tougne C., Rochat A.F., Bossen C., Schneider P., Huard B., Lambert P.H., Siegrist C.A. APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early-life bone marrow stromal cells. Blood. 2008;111:2755–2764. - PubMed
-
- Benson M.J., Dillon S.R., Castigli E., Geha R.S., Xu S., Lam K.-P., Noelle R.J. Cutting edge: the dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J. Immunol. 2008;180:3655–3659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

