Ion Channels as Therapeutic Targets for Viral Infections: Further Discoveries and Future Perspectives
- PMID: 32756358
- PMCID: PMC7472218
- DOI: 10.3390/v12080844
Ion Channels as Therapeutic Targets for Viral Infections: Further Discoveries and Future Perspectives
Abstract
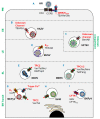
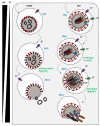
Ion channels play key roles in almost all facets of cellular physiology and have emerged as key host cell factors for a multitude of viral infections. A catalogue of ion channel-blocking drugs have been shown to possess antiviral activity, some of which are in widespread human usage for ion channel-related diseases, highlighting new potential for drug repurposing. The emergence of ion channel-virus interactions has also revealed the intriguing possibility that channelopathies may explain some commonly observed virus induced pathologies. This field is rapidly evolving and an up-to-date summary of new discoveries can inform future perspectives. We herein discuss the role of ion channels during viral lifecycles, describe the recently identified ion channel drugs that can inhibit viral infections, and highlight the potential contribution of ion channels to virus-mediated disease.
Keywords: antivirals; channelopathies; ion channel; virus; virus–host interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Netter R.C., Amberg S.M., Balliet J.W., Biscone M.J., Vermeulen A., Earp L.J., White J.M., Bates P. Heptad repeat 2-based peptides inhibit avian sarcoma and leukosis virus subgroup a infection and identify a fusion intermediate. J. Virol. 2004;78:13430–13439. doi: 10.1128/JVI.78.24.13430-13439.2004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

