Lipid Nanoparticle Acts as a Potential Adjuvant for Influenza Split Vaccine without Inducing Inflammatory Responses
- PMID: 32756368
- PMCID: PMC7565178
- DOI: 10.3390/vaccines8030433
Lipid Nanoparticle Acts as a Potential Adjuvant for Influenza Split Vaccine without Inducing Inflammatory Responses
Abstract
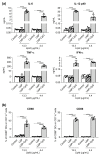
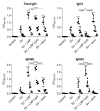
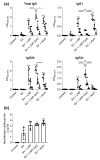
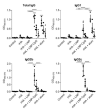
Vaccination is a critical and reliable strategy for controlling the spread of influenza viruses in populations. Conventional seasonal split vaccines (SVs) for influenza evoke weaker immune responses than other types of vaccines, such as inactivated whole-virion vaccines, although SVs are highly safe compared to other types. Here, we assessed the potential of the lipid nanoparticle (LNP) we developed as an adjuvant for conventional influenza SV as an antigen in mice. The LNP did not induce the production of cytokines such as interleukin-6 (IL-6) and IL-12 p40 by dendritic cells or the expression of co-stimulatory molecules on these cells in vitro. In contrast, an SV adjuvanted with LNP improved SV-specific IgG1 and IgG2 responses and the Th1 response compared to the SV alone in mice. In addition, SV adjuvanted with an LNP gave superior protection against the influenza virus challenge over the SV alone and was as effective as SV adjuvanted with aluminum salts in mice. The LNP did not provoke inflammatory responses such as inflammatory cytokine production and inflammatory immune cell infiltration in mice, whereas aluminum salts induced inflammatory responses. These results suggest the potential of the LNP as an adjuvant without inflammatory responses for influenza SVs. Our strategy should be useful for developing influenza vaccines with enhanced efficacy and safety.
Keywords: adjuvant; dendritic cell; inflammation; influenza virus; lipid nanoparticle; vaccine.
Conflict of interest statement
Y.Y. is employed by The Research Foundation for Microbial Diseases at Osaka University. The other authors have no conflicts of interests to declare.
Figures




References
-
- Sekiya T., Mifsud E.J., Ohno M., Nomura N., Sasada M., Fujikura D., Daito T., Shingai M., Ohara Y., Nishimura T., et al. Inactivated whole virus particle vaccine with potent immunogenicity and limited il-6 induction is ideal for influenza. Vaccine. 2019;37:2158–2166. doi: 10.1016/j.vaccine.2019.02.057. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

