Inhibition of Colony-Stimulating Factor 1 Receptor by PLX3397 Prevents Amyloid Beta Pathology and Rescues Dopaminergic Signaling in Aging 5xFAD Mice
- PMID: 32756440
- PMCID: PMC7432084
- DOI: 10.3390/ijms21155553
Inhibition of Colony-Stimulating Factor 1 Receptor by PLX3397 Prevents Amyloid Beta Pathology and Rescues Dopaminergic Signaling in Aging 5xFAD Mice
Abstract
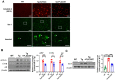
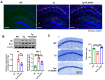
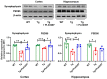
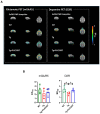
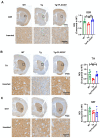
Alzheimer's disease (AD) is a progressive neurodegenerative disease. In this study, to investigate the effect of microglial elimination on AD progression, we administered PLX3397, a selective colony-stimulating factor 1 receptor inhibitor, to the mouse model of AD (5xFAD mice). Amyloid-beta (Aβ) deposition and amyloid precursor protein (APP), carboxyl-terminal fragment β, ionized calcium-binding adaptor molecule 1, synaptophysin, and postsynaptic density (PSD)-95 levels were evaluated in the cortex and hippocampus. In addition, the receptor density changes in dopamine D2 receptor (D2R) and metabotropic glutamate receptor 5 were evaluated using positron emission tomography (PET). D2R, tyrosine hydroxylase (TH), and dopamine transporter (DAT) levels were analyzed in the brains of Tg (5xFAD) mice using immunohistochemistry. PLX3397 administration significantly decreased Aβ deposition following microglial depletion in the cortex and hippocampus of Tg mice. In the neuro-PET studies, the binding values for D2R in the Tg mice were lower than those in the wild type mice; however, after PLX3397 treatment, the binding dramatically increased. PLX3397 administration also reversed the changes in synaptophysin and PSD-95 expression in the brain. Furthermore, the D2R and TH expression in the brains of Tg mice was significantly lower than that in the wild type; however, after PLX3397 administration, the D2R and TH levels were significantly higher than those in untreated Tg mice. Thus, our findings show that administering PLX3397 to aged 5xFAD mice could prevent amyloid pathology, concomitant with the rescue of dopaminergic signaling, suggesting that targeting microglia may serve as a useful therapeutic option for neurodegenerative diseases, including AD.
Keywords: Alzheimer’s disease; Alzheimer’s disease mice; Aβ pathology; PLX3397; colony-stimulating factor 1 receptor inhibitor; dopamine D2 receptor; synaptic change.
Conflict of interest statement
The authors declare no conflict of interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

