Synaptic Organization of the Human Temporal Lobe Neocortex as Revealed by High-Resolution Transmission, Focused Ion Beam Scanning, and Electron Microscopic Tomography
- PMID: 32756507
- PMCID: PMC7432700
- DOI: 10.3390/ijms21155558
Synaptic Organization of the Human Temporal Lobe Neocortex as Revealed by High-Resolution Transmission, Focused Ion Beam Scanning, and Electron Microscopic Tomography
Abstract
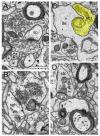
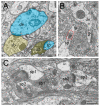
Modern electron microscopy (EM) such as fine-scale transmission EM, focused ion beam scanning EM, and EM tomography have enormously improved our knowledge about the synaptic organization of the normal, developmental, and pathologically altered brain. In contrast to various animal species, comparably little is known about these structures in the human brain. Non-epileptic neocortical access tissue from epilepsy surgery was used to generate quantitative 3D models of synapses. Beside the overall geometry, the number, size, and shape of active zones and of the three functionally defined pools of synaptic vesicles representing morphological correlates for synaptic transmission and plasticity were quantified. EM tomography further allowed new insights in the morphological organization and size of the functionally defined readily releasable pool. Beside similarities, human synaptic boutons, although comparably small (approximately 5 µm), differed substantially in several structural parameters, such as the shape and size of active zones, which were on average 2 to 3-fold larger than in experimental animals. The total pool of synaptic vesicles exceeded that in experimental animals by approximately 2 to 3-fold, in particular the readily releasable and recycling pool by approximately 2 to 5-fold, although these pools seemed to be layer-specifically organized. Taken together, synaptic boutons in the human temporal lobe neocortex represent unique entities perfectly adapted to the "job" they have to fulfill in the circuitry in which they are embedded. Furthermore, the quantitative 3D models of synaptic boutons are useful to explain and even predict the functional properties of synaptic connections in the human neocortex.
Keywords: EM tomography; active zones; human temporal lobe neocortex; quantitative three-dimensional models of synaptic boutons; synaptic boutons; synaptic vesicles; transmission and focused ion beam scanning EM.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Von Economo C., Koskinas G.N. Die Cytoarchitektonik der Hirnrinde des Erwachsenen Menschen. Verlag von Julius Springer; Wien, Austria: Berlin, Germany: 1925.
-
- Brodmann K. Beiträge zur histologischen lokalisation der grosshirnrinde. I. Mitteilung: Die regio rolandica. J. Psychol. Neurol. (Leipzig) 1903;2:79–107.
MeSH terms
LinkOut - more resources
Full Text Sources

