Multimodality approach to the nipple-areolar complex: a pictorial review and diagnostic algorithm
- PMID: 32757082
- PMCID: PMC7406635
- DOI: 10.1186/s13244-020-00896-1
Multimodality approach to the nipple-areolar complex: a pictorial review and diagnostic algorithm
Abstract
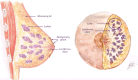
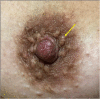
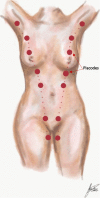
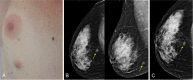
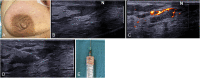
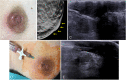
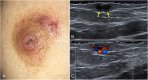
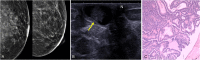
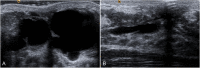
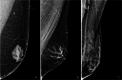
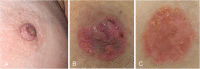
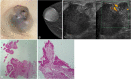
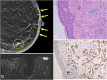
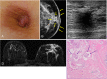
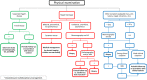
The anatomic and histologic characteristics of the nipple-areolar complex make this breast region special. The nipple-areolar complex can be affected by abnormal development and a wide spectrum of pathological conditions, many of which have unspecific clinical and radiological presentations that can present a challenge for radiologists. The nipple-areolar complex requires a specific imaging workup in which a multimodal approach is essential. Radiologists need to know the different imaging modalities used to study the nipple-areolar complex, as well as their advantages and limitations. It is essential to get acquainted with the acquisition technique for each modality and the spectrum of findings for the different conditions. This review describes and illustrates a combined clinical and radiological approach to evaluate the nipple-areolar complex, emphasizing the findings for the normal morphology, developmental abnormalities, and the most common benign and malignant diseases that can affect this region. We also present a diagnostic algorithm that enables a rapid, practical approach to diagnosing condition involving the nipple-areolar complex.
Keywords: Breast disease; Contrast-enhanced magnetic resonance imaging; Mammography; Nipple-areolar complex; Sonography.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


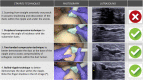
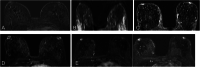
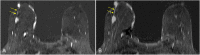
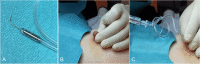
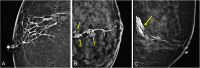

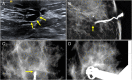

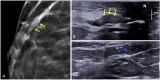
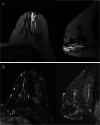
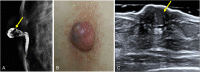
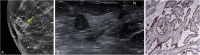
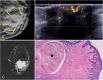
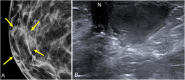



References
Publication types
LinkOut - more resources
Full Text Sources

