Photochemically Induced Ring Opening of Spirocyclopropyl Oxindoles: Evidence for a Triplet 1,3-Diradical Intermediate and Deracemization by a Chiral Sensitizer
- PMID: 32757341
- PMCID: PMC7756555
- DOI: 10.1002/anie.202008384
Photochemically Induced Ring Opening of Spirocyclopropyl Oxindoles: Evidence for a Triplet 1,3-Diradical Intermediate and Deracemization by a Chiral Sensitizer
Abstract
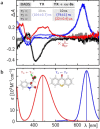
The photochemical deracemization of spiro[cyclopropane-1,3'-indolin]-2'-ones (spirocyclopropyl oxindoles) was studied. The corresponding 2,2-dichloro compound is configurationally labile upon direct irradiation at λ=350 nm and upon irradiation at λ=405 nm in the presence of achiral thioxanthen-9-one as the sensitizer. The triplet 1,3-diradical intermediate generated in the latter reaction was detected by transient absorption spectroscopy and its lifetime determined (τ=22 μs). Using a chiral thioxanthone or xanthone, with a lactam hydrogen bonding site as a photosensitizer, allowed the deracemization of differently substituted chiral spirocyclopropyl oxindoles with yields of 65-98 % and in 50-85 % ee (17 examples). Three mechanistic contributions were identified to co-act favorably for high enantioselectivity: the difference in binding constants to the chiral thioxanthone, the smaller molecular distance in the complex of the minor enantiomer, and the lifetime of the intermediate 1,3-diradical.
Keywords: enantioselectivity; hydrogen bonds; photochemistry; time-resolved spectroscopy; xanthones.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- None
-
- Klán P., Wirz J., Photochemistry of Organic Compounds, Wiley, Chichester, 2009;
-
- Turro N. J., Ramamurthy V., Scaiano J., Modern Molecular Photochemistry of Organic Molecules, University Science Books, Sausalito, 2010.
-
- For reviews covering enantioselective photochemical reactions, see:
-
- Zhou Q.-Q., Zou Y.-Q., Lu L.-Q., Xiao W.-J., Angew. Chem. Int. Ed. 2019, 58, 1586–1604; - PubMed
- Angew. Chem. 2019, 131, 1600–1619;
Publication types
LinkOut - more resources
Full Text Sources

