Main Group Multiple Bonds for Bond Activations and Catalysis
- PMID: 32757381
- PMCID: PMC7894548
- DOI: 10.1002/chem.202002939
Main Group Multiple Bonds for Bond Activations and Catalysis
Abstract
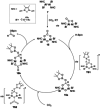
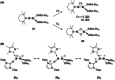
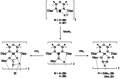
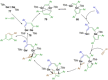
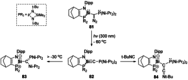
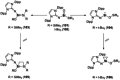
Since the discovery that the so-called "double-bond" rule could be broken, the field of molecular main group multiple bonds has expanded rapidly. With the majority of homodiatomic double and triple bonds realised within the p-block, along with many heterodiatomic combinations, this Minireview examines the reactivity of these compounds with a particular emphasis on small molecule activation. Furthermore, whilst their ability to act as transition metal mimics has been explored, their catalytic behaviour is somewhat limited. This Minireview aims to highlight the potential of these complexes towards catalytic application and their role as synthons in further functionalisations making them a versatile tool for the modern synthetic chemist.
Keywords: bond activation; catalysis; main group; multiple bonds; small molecule activation.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


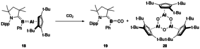

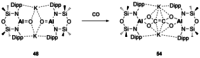
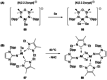
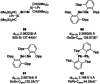


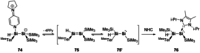



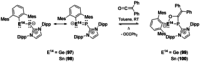


References
-
- West R., Fink M. J., Michl J., Science 1981, 214, 1343–1344. - PubMed
-
- Yoshifuji M., Shima I., Inamoto N., Hirotsu K., Higuchi T., J. Am. Chem. Soc. 1981, 103, 4587–4589.
-
- Brook A. G., Abdesaken F., Gutekunst B., Gutekunst G., Kallury R. K., J. Chem. Soc. Chem. Commun. 1981, 191–192.
-
- Becker G., Gresser G., Uhl W., Z. Naturforsch. B 1981, 36, 16–19.
-
- Power P. P., Chem. Rev. 1999, 99, 3463–3504. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

