Procalcitonin to Reduce Long-Term Infection-associated Adverse Events in Sepsis. A Randomized Trial
- PMID: 32757963
- PMCID: PMC7874409
- DOI: 10.1164/rccm.202004-1201OC
Procalcitonin to Reduce Long-Term Infection-associated Adverse Events in Sepsis. A Randomized Trial
Abstract
Rationale: Although early antimicrobial discontinuation guided by procalcitonin (PCT) has shown decreased antibiotic consumption in lower respiratory tract infections, the outcomes in long-term sepsis sequelae remain unclear.Objectives: To investigate if PCT guidance may reduce the incidence of long-term infection-associated adverse events in sepsis.Methods: In this multicenter trial, 266 patients with sepsis (by Sepsis-3 definitions) with lower respiratory tract infections, acute pyelonephritis, or primary bloodstream infection were randomized (1:1) to receive either PCT-guided discontinuation of antimicrobials or standard of care. The discontinuation criterion was ≥80% reduction in PCT levels or any PCT ≤0.5 μg/L at Day 5 or later. The primary outcome was the rate of infection-associated adverse events at Day 180, a composite of the incidence of any new infection by Clostridioides difficile or multidrug-resistant organisms, or any death attributed to baseline C. difficile or multidrug-resistant organism infection. Secondary outcomes included 28-day mortality, length of antibiotic therapy, and cost of hospitalization.Measurements and Main Results: The rate of infection-associated adverse events was 7.2% (95% confidence interval [CI], 3.8-13.1%; 9/125) versus 15.3% (95% CI, 10.1-22.4%; 20/131) (hazard ratio, 0.45; 95% CI, 0.20-0.98; P = 0.045); 28-day mortality 15.2% (95% CI, 10-22.5%; 19/125) versus 28.2% (95% CI, 21.2-36.5%; 37/131) (hazard ratio, 0.51; 95% CI, 0.29-0.89; P = 0.02); and median length of antibiotic therapy 5 (range, 5-7) versus 10 (range, 7-15) days (P < 0.001) in the PCT and standard-of-care arms, respectively. The cost of hospitalization was also reduced in the PCT arm.Conclusions: In sepsis, PCT guidance was effective in reducing infection-associated adverse events, 28-day mortality, and cost of hospitalization.Clinical trial registered with www.clinicaltrials.gov (NCT03333304).
Keywords: mortality; multidrug-resistant; procalcitonin; sepsis.
Figures
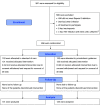
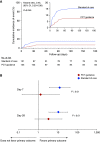
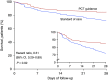
Comment in
-
Antibiotic Use in Sepsis: How and Why Less Can Really Mean More (Survival).Am J Respir Crit Care Med. 2021 Jan 15;203(2):157-158. doi: 10.1164/rccm.202008-3294ED. Am J Respir Crit Care Med. 2021. PMID: 32936687 Free PMC article. No abstract available.
References
-
- European Centre for Disease Prevention and Control. US CDC report on antibiotic resistance threats in the United States. 2013 ECDC comment. [accessed 2019 Sep 23]. Available from: https://ecdc.europa.eu/en/news-events/us-cdc-report-antibiotic-resistanc....
-
- Peters L, Olson L, Khu DTK, Linnros S, Le NK, Hanberger H, et al. Multiple antibiotic resistance as a risk factor for mortality and prolonged hospital stay: a cohort study among neonatal intensive care patients with hospital-acquired infections caused by gram-negative bacteria in Vietnam. PLoS One. 2019;14:e0215666. - PMC - PubMed
-
- Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006;42:S82–S89. - PubMed
-
- Schuetz P, Beishuizen A, Broyles M, Ferrer R, Gavazzi G, Gluck EH, et al. Procalcitonin (PCT)-guided antibiotic stewardship: an international experts consensus on optimized clinical use. Clin Chem Lab Med. 2019;57:1308–1318. - PubMed
-
- Schuetz P, Christ-Crain M, Thomann R, Falconnier C, Wolbers M, Widmer I, et al. ProHOSP Study Group. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302:1059–1066. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

