Neural Stem Cell Grafts Form Extensive Synaptic Networks that Integrate with Host Circuits after Spinal Cord Injury
- PMID: 32758426
- PMCID: PMC7484050
- DOI: 10.1016/j.stem.2020.07.007
Neural Stem Cell Grafts Form Extensive Synaptic Networks that Integrate with Host Circuits after Spinal Cord Injury
Abstract
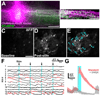
Neural stem/progenitor cell (NSPC) grafts can integrate into sites of spinal cord injury (SCI) and generate neuronal relays across lesions that can provide functional benefit. To determine if and how grafts become synaptically organized and connect with host systems, we performed calcium imaging of NSPC grafts in SCI sites in vivo and in adult spinal cord slices. NSPC grafts organize into localized and spontaneously active synaptic networks. Optogenetic stimulation of host corticospinal tract axons regenerating into grafts elicited distinct and segregated neuronal network responses throughout the graft. Moreover, optogenetic stimulation of graft-derived axons extending from the graft into the denervated spinal cord also triggered local host neuronal network responses. In vivo imaging revealed that behavioral stimulation likewise elicited focal synaptic responses within grafts. Thus neural progenitor grafts can form functional synaptic subnetworks whose activity patterns resemble intact spinal cord.
Keywords: all-optical; calcium imaging; graft; neural progenitor cell; neural stem cell; neuronal relay; optogenetics; slice preparation; spinal cord injury; transplant.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

