Apolipoprotein E isoforms differentially regulate matrix metallopeptidase 9 function in Alzheimer's disease
- PMID: 32758917
- PMCID: PMC7609500
- DOI: 10.1016/j.neurobiolaging.2020.06.018
Apolipoprotein E isoforms differentially regulate matrix metallopeptidase 9 function in Alzheimer's disease
Abstract
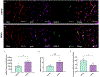
Apolipoprotein E (APOE) has been shown to influence amyloid-β (Aβ) clearance from the brain in an isoform-specific manner. Our prior work showed that Aβ transit across the blood-brain-barrier was reduced by apoE4, compared to other apoE isoforms, due to elevated lipoprotein receptor shedding in brain endothelia. Recently, we demonstrated that matrix metallopeptidase 9 (MMP-9) induces lipoprotein receptor proteolysis in an apoE isoform-dependent manner, which impacts Aβ elimination from the brain. The current studies interrogated the relationship between apoE and MMP-9 and found that apoE impacted proMMP-9 cellular secretion from brain endothelia (apoE2 < apoE3 = apoE4). In a cell-free assay, apoE dose-dependently reduced MMP-9 activity, with apoE4 showing a significantly weaker ability to inhibit MMP-9 function than apoE2 or apoE3. Finally, we observed elevated MMP-9 expression and activity in the cerebrovasculature of both human and animal AD brain specimens with an APOE4 genotype. Collectively, these findings suggest a role for apoE in regulating MMP-9 disposition and may describe the effect of apoE4 on Aβ pathology in the AD brain.
Keywords: Alzheimer’s disease; Apolipoprotein E; Binding affinity; Cerebrovasculature; Enzyme regulation; Matrix metallopeptidase 9.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures
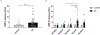
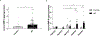
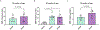


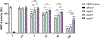

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

