Inhibition of DDR1 enhances in vivo chemosensitivity in KRAS-mutant lung adenocarcinoma
- PMID: 32759499
- PMCID: PMC7455065
- DOI: 10.1172/jci.insight.137869
Inhibition of DDR1 enhances in vivo chemosensitivity in KRAS-mutant lung adenocarcinoma
Abstract
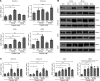
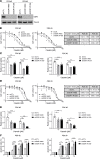
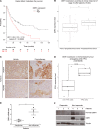
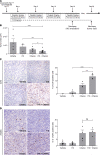
Platinum-based chemotherapy in combination with immune-checkpoint inhibitors is the current standard of care for patients with advanced lung adenocarcinoma (LUAD). However, tumor progression evolves in most cases. Therefore, predictive biomarkers are needed for better patient stratification and for the identification of new therapeutic strategies, including enhancing the efficacy of chemotoxic agents. Here, we hypothesized that discoidin domain receptor 1 (DDR1) may be both a predictive factor for chemoresistance in patients with LUAD and a potential target positively selected in resistant cells. By using biopsies from patients with LUAD, KRAS-mutant LUAD cell lines, and in vivo genetically engineered KRAS-driven mouse models, we evaluated the role of DDR1 in the context of chemotherapy treatment. We found that DDR1 is upregulated during chemotherapy both in vitro and in vivo. Moreover, analysis of a cohort of patients with LUAD suggested that high DDR1 levels in pretreatment biopsies correlated with poor response to chemotherapy. Additionally, we showed that combining DDR1 inhibition with chemotherapy prompted a synergistic therapeutic effect and enhanced cell death of KRAS-mutant tumors in vivo. Collectively, this study suggests a potential role for DDR1 as both a predictive and prognostic biomarker, potentially improving the chemotherapy response of patients with LUAD.
Keywords: Drug therapy; Lung cancer; Molecular biology; Oncology; Therapeutics.
Conflict of interest statement
Figures