Genoprotective Properties and Metabolites of β-Glucan-Rich Edible Mushrooms Following Their In Vitro Fermentation by Human Faecal Microbiota
- PMID: 32759726
- PMCID: PMC7435999
- DOI: 10.3390/molecules25153554
Genoprotective Properties and Metabolites of β-Glucan-Rich Edible Mushrooms Following Their In Vitro Fermentation by Human Faecal Microbiota
Erratum in
-
Correction: Boulaka et al. Genoprotective Properties and Metabolites of β-Glucan-Rich Edible Mushrooms Following Their In Vitro Fermentation by Human Faecal Microbiota. Molecules 2020, 25, 3554.Molecules. 2023 Jul 11;28(14):5337. doi: 10.3390/molecules28145337. Molecules. 2023. PMID: 37513492 Free PMC article.
Abstract
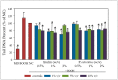
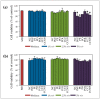
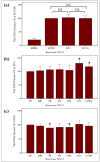
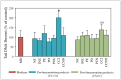
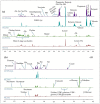
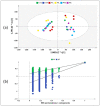
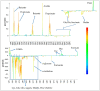
A variety of bioactive compounds, constituents of edible mushrooms, in particular β-glucans, i.e., a group of β-d-glucose polysaccharides abundant in the fungal cell walls, have been linked to immunomodulating, anticancer and prebiotic activities. The aim of the study was the investigation of the genoprotective effects of edible mushrooms produced by Pleurotus eryngii, Pleurotus ostreatus and Cyclocybe cylindracea (Basidiomycota). Mushrooms from selected strains of the species mentioned above were fermented in vitro using faecal inocula from healthy volunteers. The cytotoxic and anti-genotoxic properties of the fermentation supernatants (FSs) were investigated in Caco-2 human colon adenocarcinoma cells. The FSs were cytotoxic in a dose-dependent manner. Non-cytotoxic concentrations were used for the genotoxicity studies, which revealed that mushrooms' FSs have the ability to protect Caco-2 cells against tert-butyl hydroperoxide (t-BOOH), a known genotoxic agent. Their global metabolic profiling was assessed by 1H-NMR spectroscopy. A total of 37 metabolites were identified with the use of two-dimensional (2D) homo- and hetero-nuclear NMR experiments. Multivariate data analysis monitored the metabolic variability of gut microbiota and probed to biomarkers potentially associated with the health-promoting effects of edible mushrooms.
Keywords: NMR-based metabolomics; edible mushrooms; faecal microbiota; genoprotection; in vitro fermentation; β-glucans.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Gargano M.L., van Griensven L.J., Isikhuemhen O.S., Lindequist U., Venturella G., Wasser S.P., Zervakis G.I. Medicinal mushrooms: Valuable biological resources of high exploitation potential. Plant Biosyst. 2017;151:548–565. doi: 10.1080/11263504.2017.1301590. - DOI
-
- Wasser S.P., Weis A.L. Therapeutic effects of substances occurring in higher Basidiomycetes mushrooms: A modern perspective. Crit. Rev. Immunol. 1999;19:65–96. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

