Advances with RNAi-Based Therapy for Hepatitis B Virus Infection
- PMID: 32759756
- PMCID: PMC7472220
- DOI: 10.3390/v12080851
Advances with RNAi-Based Therapy for Hepatitis B Virus Infection
Abstract
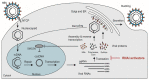
Infection with hepatitis B virus (HBV) remains a global health challenge. Approximately 292 million people worldwide are chronically infected with HBV and the annual mortality from the infection is approaching 900,000. Despite the availability of an effective prophylactic vaccine, millions of individuals are at risk of potentially fatal complicating cirrhosis and hepatocellular carcinoma. Current drug treatments can suppress viral replication, slow the progression of liver fibrosis, and reduce infectivity, but can rarely clear the viral covalently closed circular DNA (cccDNA) that is responsible for HBV persistence. Alternative therapeutic strategies, including those based on viral gene silencing by harnessing the RNA interference (RNAi) pathway, effectively suppress HBV replication and thus hold promise. RNAi-based silencing of certain viral genes may even lead to disabling of cccDNA during chronic infection. This review summarizes different RNAi activators that have been tested against HBV, the advances with vectors used to deliver artificial potentially therapeutic RNAi sequences to the liver, and the current status of preclinical and clinical investigation.
Keywords: HBV; RNAi; cccDNA; miRNA; shRNA; siRNA.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the writing of the manuscript.
Figures

References
-
- Steven A.C., Conway J.F., Cheng N., Watts N.R., Belnap D.M., Harris A., Stahl S.J., Wingfield P.T. Structure, assembly, and antigenicity of hepatitis B virus capsid proteins. Adv. Virus Res. 2005;64:125–164. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

