Endocytosis: A Turnover Mechanism Controlling Ion Channel Function
- PMID: 32759790
- PMCID: PMC7463639
- DOI: 10.3390/cells9081833
Endocytosis: A Turnover Mechanism Controlling Ion Channel Function
Abstract
Ion channels (IChs) are transmembrane proteins that selectively drive ions across membranes. The function of IChs partially relies on their abundance and proper location in the cell, fine-tuned by the delicate balance between secretory, endocytic, and degradative pathways. The disruption of this balance is associated with several diseases, such as Liddle's and long QT syndromes. Because of the vital role of these proteins in human health and disease, knowledge of ICh turnover is essential. Clathrin-dependent and -independent mechanisms have been the primary mechanisms identified with ICh endocytosis and degradation. Several molecular determinants recognized by the cellular internalization machinery have been discovered. Moreover, specific conditions can trigger the endocytosis of many IChs, such as the activation of certain receptors, hypokalemia, and some drugs. Ligand-dependent receptor activation primarily results in the posttranslational modification of IChs and the recruitment of important mediators, such as β-arrestins and ubiquitin ligases. However, endocytosis is not a final fate. Once internalized into endosomes, IChs are either sorted to lysosomes for degradation or recycled back to the plasma membrane. Rab proteins are crucial participants during these turnover steps. In this review, we describe the major ICh endocytic pathways, the signaling inputs triggering ICh internalization, and the key mediators of this essential cellular process.
Keywords: endocytosis; ion channels; turnover; ubiquitination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
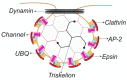
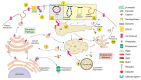
References
-
- Hille B. Ion. Channels of Excitable Membranes. 3rd ed. Sinauer Associates, INC; Sunderland, MA, USA: 2001.
-
- Zheng J., Trudeau M.C. Handbook of Ion Channels. Taylor & Francis Goup, LLC; Boca Raton, FL, USA: 2015.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

