Infection during the first year in patients treated with CD19 CAR T cells for diffuse large B cell lymphoma
- PMID: 32759935
- PMCID: PMC7405315
- DOI: 10.1038/s41408-020-00346-7
Infection during the first year in patients treated with CD19 CAR T cells for diffuse large B cell lymphoma
Abstract
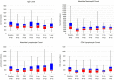
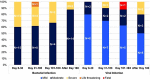
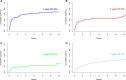
CD19-targeted chimeric antigen receptor (CAR) T cell therapy is an effective treatment for diffuse large B cell lymphoma (DLBCL). In addition to cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity (ICANS), B cell aplasia and hypogammaglobulinemia are common toxicities predisposing these patients to infections. We analyzed 60 patients with DLBCL treated with FDA-approved CD19 CAR T cells and report the incidence, risk factors, and management of infections during the first year after treatment. A total of 101 infectious events were observed, including 25 mild, 51 moderate, 23 severe, 1 life-threatening, and 1 fatal infection. Bacteria were the most common causative pathogens. The cumulative incidence of overall, bacterial, severe bacterial, viral, and fungal infection at 1 year were 63.3%, 57.2%, 29.6%, 44.7%, and 4%, respectively. In multivariate analyses, the use of systemic corticosteroids for the management of CRS or ICANS was associated with an increased risk of infections and prolonged admission. Impaired performance status and history of infections within 30 days before CAR T cell therapy was a risk factor for severe bacterial infection. In conclusion, infections were common within the first 60 days after CAR T cell therapy, however, they were not associated with an increased risk of death.
Conflict of interest statement
K.W., M.P., J.R.F., S.M.D., A.A., M.L.S., M.A.M., and S.K.S. have no relevant conflict of interest. M.G.R. reports receiving honoraria from Takeda and Janssen Pharmaceutical. M.S. has served as a paid consultant for McKinsey & Company, Angiocrine Bioscience, Inc., and Omeros Corporation. He has received research funding from Angiocrine Bioscience, Inc. He has served on an ad hoc advisory board for Kite—A Gilead Company. G.L.S. receives research funding from Amgen and Janssen Pharmaceutical. C.L.B. serves as a paid consultant for Life Sci, GLG, Juno/Celgene, Seattle Genetics and Kite/Gilead. She reports receiving research funding from Janssen Pharmaceutical, Novartis, Epizyme, Xynomics and Bayer. She receives honorarium from Dava Oncology. M.L.P. has served on ad hoc advisory board for Kite and Novartis. P.B.D. serves on the advisory board for Kite/Gilead. C.S.S. has served as a paid consultant on advisory boards for Juno Therapeutics, Sanofi-Genzyme, Spectrum Pharmaceuticals, Novartis, Genmab, Precision Biosciences, Kite, a Gilead Company, Celgene, Gamida Cell and GSK. He has received research funds for clinical trials from Juno Therapeutics, Celgene, Precision Biosciences and Sanofi-Genzyme. B.D.S. provides consultancy for Kite/Gilead, Juno/Celgene and Janssen. She also receives research support from ADC Therapeutics. M.A.P. reports honoraria from Kite/Gilead, Abbvie, Bellicum, Bristol-Myers Squibb, Incyte, Merck, Novartis, Nektar Therapeutics, Omeros, and Takeda. He serves on DSMBs for Servier and Medigene, and the scientific advisory boards of MolMed and NexImmune. He has received research support for clinical trials from Incyte, Kite/Gilead and Miltenyi Biotec. He serves in a volunteer capacity as a member of the Board of Directors of the American Society for Transplantation and Cellular Therapy (ASTCT) and Be the Match (National Marrow Donor Program, NMDP), as well as on the CIBMTR Cellular Immunotherapy Data Resource (CIDR) Committee.
Figures
References
-
- Jain T, et al. Use of chimeric antigen receptor T cell therapy in clinical practice for relapsed/refractory aggressive B cell non-Hodgkin lymphoma: an expert panel opinion from the american society for transplantation and cellular therapy. Biol Blood Marrow Transplant. 2019;25:2305–2321. doi: 10.1016/j.bbmt.2019.08.015. - DOI - PubMed
-
- Nahas G, et al. Persistent cytopenias after chimeric antigen receptor T-cell immunotherapy for CD19+ aggressive lymphoma: a single institution experience. Biol. Blood Marrow Transplant. 2019;25:S180. doi: 10.1016/j.bbmt.2018.12.324. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

