ROS1-dependent cancers - biology, diagnostics and therapeutics
- PMID: 32760015
- PMCID: PMC8830365
- DOI: 10.1038/s41571-020-0408-9
ROS1-dependent cancers - biology, diagnostics and therapeutics
Abstract
The proto-oncogene ROS1 encodes a receptor tyrosine kinase with an unknown physiological role in humans. Somatic chromosomal fusions involving ROS1 produce chimeric oncoproteins that drive a diverse range of cancers in adult and paediatric patients. ROS1-directed tyrosine kinase inhibitors (TKIs) are therapeutically active against these cancers, although only early-generation multikinase inhibitors have been granted regulatory approval, specifically for the treatment of ROS1 fusion-positive non-small-cell lung cancers; histology-agnostic approvals have yet to be granted. Intrinsic or extrinsic mechanisms of resistance to ROS1 TKIs can emerge in patients. Potential factors that influence resistance acquisition include the subcellular localization of the particular ROS1 oncoprotein and the TKI properties such as the preferential kinase conformation engaged and the spectrum of targets beyond ROS1. Importantly, the polyclonal nature of resistance remains underexplored. Higher-affinity next-generation ROS1 TKIs developed to have improved intracranial activity and to mitigate ROS1-intrinsic resistance mechanisms have demonstrated clinical efficacy in these regards, thus highlighting the utility of sequential ROS1 TKI therapy. Selective ROS1 inhibitors have yet to be developed, and thus the specific adverse effects of ROS1 inhibition cannot be deconvoluted from the toxicity profiles of the available multikinase inhibitors. Herein, we discuss the non-malignant and malignant biology of ROS1, the diagnostic challenges that ROS1 fusions present and the strategies to target ROS1 fusion proteins in both treatment-naive and acquired-resistance settings.
Conflict of interest statement
Competing interests
A.D. has received honoraria from or participated on the advisory boards of 14ner/Elevation Oncology, Abbvie, ArcherDX, AstraZeneca, Beigene, BergenBio, Blueprint Medicines, Exelixis, Helsinn, Hengrui Therapeutics, Ignyta/Genentech/Roche, Loxo/Bayer/Lilly, Monopteros, MORE Health, Pfizer, Remedica, Takeda/Ariad/Millenium, TP Therapeutics, Tyra Biosciences and Verastem; research support paid to his institution from Exelixis, GlaxoSmithKlein, Pfizer, PharmaMar, Taiho and Teva; research support from Foundation Medicine; personal fees from Boehringer Ingelheim, Merck, Merus and Puma; and CME honoraria from Axis, Medscape, OncLive, Paradigm Medical Communications, Peerview Institute, PeerVoice, Physicians Education Resources, Research to Practice, Targeted Oncology and WebMD. The other authors declare no competing interests.
Figures


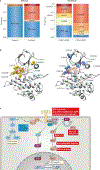


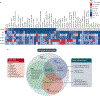
References
-
- Sharma S et al. Characterization of the ros1-gene products expressed in human glioblastoma cell lines. Oncogene Res 5, 91–100 (1989). - PubMed
-
- Charest A et al. Fusion of FIG to the receptor tyrosine kinase ROS in a glioblastoma with an interstitial del(6) (q21q21). Genes Chromosomes Cancer 37, 58–71 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

