Focused Ultrasound for Noninvasive, Focal Pharmacologic Neurointervention
- PMID: 32760238
- PMCID: PMC7372945
- DOI: 10.3389/fnins.2020.00675
Focused Ultrasound for Noninvasive, Focal Pharmacologic Neurointervention
Abstract
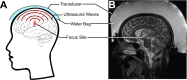
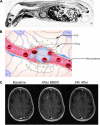
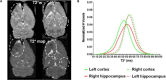
A long-standing goal of translational neuroscience is the ability to noninvasively deliver therapeutic agents to specific brain regions with high spatiotemporal resolution. Focused ultrasound (FUS) is an emerging technology that can noninvasively deliver energy up the order of 1 kW/cm2 with millimeter and millisecond resolution to any point in the human brain with Food and Drug Administration-approved hardware. Although FUS is clinically utilized primarily for focal ablation in conditions such as essential tremor, recent breakthroughs have enabled the use of FUS for drug delivery at lower intensities (i.e., tens of watts per square centimeter) without ablation of the tissue. In this review, we present strategies for image-guided FUS-mediated pharmacologic neurointerventions. First, we discuss blood-brain barrier opening to deliver therapeutic agents of a variety of sizes to the central nervous system. We then describe the use of ultrasound-sensitive nanoparticles to noninvasively deliver small molecules to millimeter-sized structures including superficial cortical regions and deep gray matter regions within the brain without the need for blood-brain barrier opening. We also consider the safety and potential complications of these techniques, with attention to temporal acuity. Finally, we close with a discussion of different methods for mapping the ultrasound field within the brain and describe future avenues of research in ultrasound-targeted drug therapies.
Keywords: blood–brain barrier; drug delivery; focused ultrasound; nanotechnology; neurointervention; neuromodulation.
Copyright © 2020 Wang, Di Ianni, Vyas, Huang, Park, Hosseini-Nassab, Aryal and Airan.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

