Safety and efficacy of sodium carboxymethyl cellulose for all animal species
- PMID: 32760467
- PMCID: PMC7393346
- DOI: 10.2903/j.efsa.2020.6211
Safety and efficacy of sodium carboxymethyl cellulose for all animal species
Abstract
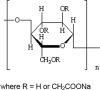
Following a request from the European Commission, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on sodium carboxymethyl cellulose as a feed additive for all animal species. Sodium carboxymethyl cellulose is intended for use as a technological additive (functional groups: emulsifier, stabiliser, thickener, gelling agent and binder) in premixtures and feedingstuffs for all animal species with no minimum and maximum content. A proper identification and characterisation of sodium carboxymethyl cellulose as required for a feed additive is not available and the occurrence of potential toxic impurities cannot be assessed. The following conclusions apply only to sodium carboxymethyl cellulose meeting the food additive specifications. The FEEDAP Panel concluded that sodium carboxymethyl cellulose is considered safe for all animal species. The use of sodium carboxymethyl cellulose in animal nutrition is of no concern for consumer safety. In the absence of data, the FEEDAP Panel was not in the position to conclude on the safety of sodium carboxymethyl cellulose for the user. The use of sodium carboxymethyl cellulose as a feed additive is considered safe for the environment. The additive is considered to be efficacious in feedingstuffs for all animal species.
Keywords: E 466; Sodium carboxymethyl cellulose; all animal species; binder; emulsifier; gelling agents; stabiliser; technological additives; thickener.
© 2020 European Food Safety Authority. EFSA Journal published by John Wiley and Sons Ltd on behalf of European Food Safety Authority.
Figures
References
-
- Bergot F and Breque J, 1983. Digestibility of starch by rainbow trout: effects of the physical state of starch and of the intake level. Aquaculture, 34, 203–212.
-
- Chiou PWS, Bi Yu B and Lin C, 1998. The effect of different fibre components on growth rate, nutrient digestibility, rate of digesta passage and hindgut fermentation in domesticated rabbits. Laboratory Animals, 32, 276–283. - PubMed
-
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), Younes M, Aggett P, Aguilar F, Crebelli R, Di Domenico A, Dusemund B, Filipič M, Jose Frutos M, Galtier P, Gott D, Gundert‐Remy U, Georg Kuhnle G, Lambré C, Leblanc J‐C, Lillegaard IT, Moldeus P, Mortensen A, Oskarsson A, Stankovic I, Tobback P, Waalkens‐Berendsen I, Wright M, Tard A, Tasiopoulou S and Woutersen RA, 2018. Scientifi c Opinion on the re‐evaluation of celluloses E 460(i), E 460(ii), E 461, E 462, E 463, E 464, E 465, E 466, E 468 and E 469 as food additives. EFSA Journal 2018;16(1):5047, 104 pp. 10.2903/j.efsa.2018.5047 - DOI - PubMed
-
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavouringsand Processing Aids), Silano V, Bolognesi C, Chipma n K, Cravedi J‐P, Engel K‐H, Fowler P, Franz R, Grob K, Gürtler R, Husøy T, Kärenlampi S, Mennes W, Milana MR, Pfaff K, Riviere G, Srinivasan J, Tavares Pocßas MF, Tlustos C, Wlöfle D, Zorn H, Kolf‐Clauw M, Lampi E, Svensson K, Van Haver E and Castle L, 2018. Scientific Opinion on the safety assessment of the active substances carboxymethyl cellulose, acetylated distarch phosphate, bentonite, boric acid and aluminium sulfate, for use in active food contact materials. EFSA Journal 2018;16(2):5121, 7 pp. 10.2903/j.efsa.2018.5121 - DOI - PMC - PubMed
-
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012a. Guidance for the preparation of dossiers for technological additives. EFSA Journal 2012;10(1):2528, 23 pp. 10.2903/j.efsa.2012.2528 - DOI