Duloxetine hydrochloride loaded film forming dermal gel enriched with methylcobalamin and geranium oil attenuates paclitaxel-induced peripheral neuropathy in rats
- PMID: 32760845
- PMCID: PMC7390834
- DOI: 10.1016/j.ibror.2020.07.006
Duloxetine hydrochloride loaded film forming dermal gel enriched with methylcobalamin and geranium oil attenuates paclitaxel-induced peripheral neuropathy in rats
Abstract
Objective: In attempt to conquer the major concerns of oral duloxetine hydrochloride (like low bioavailability, intolerable side-effects and no regeneration of demyelinated nerve fibres) for the management of chemotherapy-induced peripheral neuropathy (CIPN), an alternative delivery of duloxetine hydrochloride was aimed for in-vivo optimization.
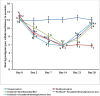
Methods: A film forming dermal gel consisting of duloxetine hydrochloride was formulated and enriched with methylcobalamin and geranium oil. The formulated gel successfully qualified the various pharmaceutical characteristics of gel. Administration of paclitaxel (8 mg/kg/i.p. in four divided doses) for 4 alternate days induced the symptoms of peripheral neuropathy in rats. On 14th day, the responses to noxious stimulus (mechanical hyperalgesia, cold allodynia, and heat hyperalgesia) were increased and reached to its maximum. Thereafter, drug treatment with formulated dermal gel and oral duloxetine hydrochloride (30 mg/kg, once daily) was initiated for 2 weeks in different group of animals. On the 28th day animals were sacrificed to isolate sciatic nerve, to assess biochemical changes (TBARS, reduced GSH, total protein, TNF-α, IL-6) and for histopathological examinations of nerve sections using Hematoxylin-Eosin and Toludine blue staining methods.
Results: Application of formulated dermal gel to paclitaxel-treated rats significantly improved paw-withdrawal latency responses during noxious stimulus testing, reduced the levels of TBARS, TNF-α, IL-6 and elevated the levels of reduced GSH as compared to paclitaxel treated rats. Histographs also indicated marked regeneration of the damaged nerve fibers. Topical delivery of duloxetine hydrochloride produced similar results in disparity to oral route. However, no significant disparity in responses was obtained with twice application of formulated dermal gel when compared to once daily application.
Conclusion: Tremendous recovery from nociception, oxidation and inflammation in addition to nerve degeneration was achieved through dermal application of duloxetine hydrochloride in peripheral neuropathy.
Keywords: Chemotherapy-induced peripheral neuropathy; Duloxetine hydrochloride; Film forming dermal gel; Methylcobalamin; Paclitaxel.
© 2020 The Author(s).
Figures





References
-
- Ahmed T.A., Khalid M. Development of alginate-reinforced chitosan nanoparticles utilizing W/O nanoemulsification/internal crosslinking technique for transdermal delivery of rabeprazole. Life Sci. 2014;110:35–43. - PubMed
-
- Argyriou A.A., Koltzenburg M., Polychronopoulos P., Papapetropoulos S., Kalofonos H.P. Peripheral nerve damage associated with administration of taxanes in patients with cancer. Crit. Rev. Oncol. Hematol. 2008;66(3):218–228. - PubMed
-
- Balayssac D., Ferrier J., Descoeur J., Ling B., Pezet D., Eschalier A. Chemotherapy-induced peripheral neuropathies: from clinical relevance to preclinical evidence. Expert Opin. Drug Saf. 2011;10(3):407–417. - PubMed
-
- Beutler E., Duron O., Kelly B.M. Improved method for the determination of blood glutathione. J. Lb. Clin. Med. 1963;61(13):882–888. - PubMed
-
- Boland B.A., Sherry V., Polomano R.C. Chemotherapy- induced peripheral neuropathy in cancer survivors. Oncol. Nurse Edn. 2010;24 33-38, 42-43.
LinkOut - more resources
Full Text Sources

