Pre-assembled Ca2+ entry units and constitutively active Ca2+ entry in skeletal muscle of calsequestrin-1 knockout mice
- PMID: 32761048
- PMCID: PMC7537346
- DOI: 10.1085/jgp.202012617
Pre-assembled Ca2+ entry units and constitutively active Ca2+ entry in skeletal muscle of calsequestrin-1 knockout mice
Abstract
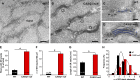
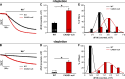
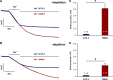
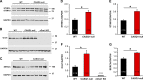
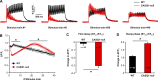
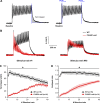
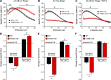
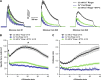
Store-operated Ca2+ entry (SOCE) is a ubiquitous Ca2+ influx mechanism triggered by depletion of Ca2+ stores from the endoplasmic/sarcoplasmic reticulum (ER/SR). We recently reported that acute exercise in WT mice drives the formation of Ca2+ entry units (CEUs), intracellular junctions that contain STIM1 and Orai1, the two key proteins mediating SOCE. The presence of CEUs correlates with increased constitutive- and store-operated Ca2+ entry, as well as sustained Ca2+ release and force generation during repetitive stimulation. Skeletal muscle from mice lacking calsequestrin-1 (CASQ1-null), the primary Ca2+-binding protein in the lumen of SR terminal cisternae, exhibits significantly reduced total Ca2+ store content and marked SR Ca2+ depletion during high-frequency stimulation. Here, we report that CEUs are constitutively assembled in extensor digitorum longus (EDL) and flexor digitorum brevis (FDB) muscles of sedentary CASQ1-null mice. The higher density of CEUs in EDL (39.6 ± 2.1/100 µm2 versus 2.0 ± 0.3/100 µm2) and FDB (16.7 ± 1.0/100 µm2 versus 2.7 ± 0.5/100 µm2) muscles of CASQ1-null compared with WT mice correlated with enhanced constitutive- and store-operated Ca2+ entry and increased expression of STIM1, Orai1, and SERCA. The higher ability to recover Ca2+ ions via SOCE in CASQ1-null muscle served to promote enhanced maintenance of peak Ca2+ transient amplitude, increased dependence of luminal SR Ca2+ replenishment on BTP-2-sensitive SOCE, and increased maintenance of contractile force during repetitive, high-frequency stimulation. Together, these data suggest that muscles from CASQ1-null mice compensate for the lack of CASQ1 and reduction in total releasable SR Ca2+ content by assembling CEUs to promote constitutive and store-operated Ca2+ entry.
© 2020 Michelucci et al.
Figures



Comment in
-
ECC meets CEU-New focus on the backdoor for calcium ions in skeletal muscle cells.J Gen Physiol. 2020 Oct 5;152(10):e202012679. doi: 10.1085/jgp.202012679. J Gen Physiol. 2020. PMID: 32851409 Free PMC article.
References
-
- Barone V., Del Re V., Gamberucci A., Polverino V., Galli L., Rossi D., Costanzi E., Toniolo L., Berti G., Malandrini A., et al. . 2017. Identification and characterization of three novel mutations in the CASQ1 gene in four patients with tubular aggregate myopathy. Hum. Mutat. 38:1761–1773. 10.1002/humu.23338 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

