Patterns of CAG repeat instability in the central nervous system and periphery in Huntington's disease and in spinocerebellar ataxia type 1
- PMID: 32761094
- PMCID: PMC7471505
- DOI: 10.1093/hmg/ddaa139
Patterns of CAG repeat instability in the central nervous system and periphery in Huntington's disease and in spinocerebellar ataxia type 1
Abstract
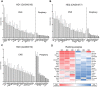
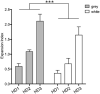
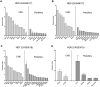
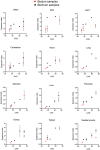
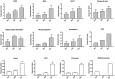
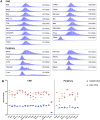
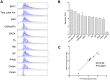
The expanded HTT CAG repeat causing Huntington's disease (HD) exhibits somatic expansion proposed to drive the rate of disease onset by eliciting a pathological process that ultimately claims vulnerable cells. To gain insight into somatic expansion in humans, we performed comprehensive quantitative analyses of CAG expansion in ~50 central nervous system (CNS) and peripheral postmortem tissues from seven adult-onset and one juvenile-onset HD individual. We also assessed ATXN1 CAG repeat expansion in brain regions of an individual with a neurologically and pathologically distinct repeat expansion disorder, spinocerebellar ataxia type 1 (SCA1). Our findings reveal similar profiles of tissue instability in all HD individuals, which, notably, were also apparent in the SCA1 individual. CAG expansion was observed in all tissues, but to different degrees, with multiple cortical regions and neostriatum tending to have the greatest instability in the CNS, and liver in the periphery. These patterns indicate different propensities for CAG expansion contributed by disease locus-independent trans-factors and demonstrate that expansion per se is not sufficient to cause cell type or disease-specific pathology. Rather, pathology may reflect distinct toxic processes triggered by different repeat lengths across cell types and diseases. We also find that the HTT CAG length-dependent expansion propensity of an individual is reflected in all tissues and in cerebrospinal fluid. Our data indicate that peripheral cells may be a useful source to measure CAG expansion in biomarker assays for therapeutic efforts, prompting efforts to dissect underlying mechanisms of expansion that may differ between the brain and periphery.
© The Author(s) 2020. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

References
-
- Vonsattel J.P., Myers R.H., Stevens T.J., Ferrante R.J., Bird E.D. and Richardson E.P. (1985) Neuropathological classification of Huntington's disease. J. Neuropathol. Exp. Neurol., 44, 559–577. - PubMed
-
- Nance M.A. and Myers R.H. (2001) Juvenile onset Huntington's disease-clinical and research perspectives. Ment. Retard. Dev. Disabil. Res. Rev., 7, 153–157. - PubMed
-
- Fusilli C., Migliore S., Mazza T., Consoli F., De Luca A., Barbagallo G., Ciammola A., Gatto E.M., Cesarini M., Etcheverry J.L. et al. (2018) Biological and clinical manifestations of juvenile Huntington's disease: a retrospective analysis. Lancet Neurol., 17, 986–993. - PubMed
-
- Cronin T., Rosser A. and Massey T. (2019) Clinical presentation and features of juvenile-onset Huntington’s disease: a systematic review. J. Huntington’s Dis., 8, 171–179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

