Evaluation of Serological Tests for SARS-CoV-2: Implications for Serology Testing in a Low-Prevalence Setting
- PMID: 32761124
- PMCID: PMC7454699
- DOI: 10.1093/infdis/jiaa467
Evaluation of Serological Tests for SARS-CoV-2: Implications for Serology Testing in a Low-Prevalence Setting
Abstract
Background: Robust serological assays are essential for long-term control of the COVID-19 pandemic. Many recently released point-of-care (PoCT) serological assays have been distributed with little premarket validation.
Methods: Performance characteristics for 5 PoCT lateral flow devices approved for use in Australia were compared to a commercial enzyme immunoassay (ELISA) and a recently described novel surrogate virus neutralization test (sVNT).
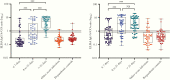
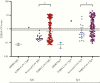
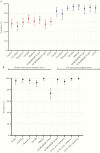
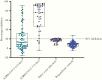
Results: Sensitivities for PoCT ranged from 51.8% (95% confidence interval [CI], 43.1%-60.4%) to 67.9% (95% CI, 59.4%-75.6%), and specificities from 95.6% (95% CI, 89.2%-98.8%) to 100.0% (95% CI, 96.1%-100.0%). ELISA sensitivity for IgA or IgG detection was 67.9% (95% CI, 59.4%-75.6%), increasing to 93.8% (95% CI, 85.0%-98.3%) for samples >14 days post symptom onset. sVNT sensitivity was 60.9% (95% CI, 53.2%-68.4%), rising to 91.2% (95% CI, 81.8%-96.7%) for samples >14 days post symptom onset, with specificity 94.4% (95% CI, 89.2%-97.5%).
Conclusions: Performance characteristics for COVID-19 serological assays were generally lower than those reported by manufacturers. Timing of specimen collection relative to onset of illness or infection is crucial in reporting of performance characteristics for COVID-19 serological assays. The optimal algorithm for implementing serological testing for COVID-19 remains to be determined, particularly in low-prevalence settings.
Keywords: COVID-19; ELISA; lateral flow; neutralization; serology.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

