Comparing Nasopharyngeal Swab and Early Morning Saliva for the Identification of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)
- PMID: 32761244
- PMCID: PMC7454325
- DOI: 10.1093/cid/ciaa1156
Comparing Nasopharyngeal Swab and Early Morning Saliva for the Identification of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)
Abstract
Background: The ideal severe acute respiratory syndrome coronavirus 2 (SARs-CoV-2) testing method would be accurate and also be patient-performed to reduce exposure to healthcare workers. The aim of this study was to compare patient-performed testing based on a morning saliva sample with the current standard testing method, healthcare worker-collected sampling via a nasopharyngeal swab (NPS).
Methods: This was a prospective single center study which recruited 217 asymptomatic adult male participants in a coronavirus disease 2019 (COVID-19) quarantine center who had tested positive for SARS-CoV-2 8-10 days prior to isolation. Paired NPS and saliva specimens were collected and processed within 5 hours of sample collection. Real time reverse transcription polymerase chain reaction (RT-PCR) targeting Envelope (E) and RNA-dependent RNA polymerase (RdRp) genes was performed and the results were compared.
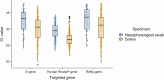
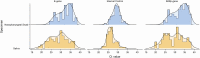
Results: Overall, 160 of the 217 (74%) participants tested positive for COVID-19 based on saliva, NPS, or both testing methods. The detection rate for SARS-CoV-2 was higher in saliva compared to NPS testing (93.1%, 149/160 vs 52.5%, 84/160, P < .001). The concordance between the 2 tests was 45.6% (virus was detected in both saliva and NPS in 73/160), whereas 47.5% were discordant (87/160 tested positive for 1 whereas negative for the other). The cycle threshold (Ct) values for E and RdRp genes were significantly lower in saliva specimens compared to NP swab specimens.
Conclusions: Our findings demonstrate that saliva is a better alternative specimen for detection of SARS-CoV-2. Taking into consideration, the simplicity of specimen collection, shortage of PPE and the transmissibility of the virus, saliva could enable self-collection for an accurate SARS-CoV-2 surveillance testing.
Keywords: COVID-19; SARS-CoV-2; nasopharyngeal swab; pandemic; saliva.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
Comment in
-
No One Likes a Stick up Their Nose: Making the Case for Saliva-based Testing for Coronavirus Disease 2019 (COVID-19).Clin Infect Dis. 2021 May 4;72(9):e357-e358. doi: 10.1093/cid/ciaa1314. Clin Infect Dis. 2021. PMID: 32875330 Free PMC article. No abstract available.
References
-
- Tharanya Arumugam RK. 325 medical workers test positive for Covid-19. News Strait Times, NST [Internet]. 2020. [cited 2020 Jul 29]. Available at: https://www.nst.com.my/news/nation/2020/04/586972/325-medical-workers-te...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

