The COVID-19 Vaccine Race: Challenges and Opportunities in Vaccine Formulation
- PMID: 32761294
- PMCID: PMC7405756
- DOI: 10.1208/s12249-020-01744-7
The COVID-19 Vaccine Race: Challenges and Opportunities in Vaccine Formulation
Abstract
In the race for a safe and effective vaccine against coronavirus disease (COVID)-19, pharmaceutical formulation science plays a critical role throughout the development, manufacturing, distribution, and vaccination phases. The proper choice of the type of vaccine, carrier or vector, adjuvant, excipients, dosage form, and route of administration can directly impact not only the immune responses induced and the resultant efficacy against COVID-19, but also the logistics of manufacturing, storing and distributing the vaccine, and mass vaccination. In this review, we described the COVID-19 vaccines that are currently tested in clinical trials and provided in-depth insight into the various types of vaccines, their compositions, advantages, and potential limitations. We also addressed how challenges in vaccine distribution and administration may be alleviated by applying vaccine-stabilization strategies and the use of specific mucosal immune response-inducing, non-invasive routes of administration, which must be considered early in the development process.
Keywords: adjuvant; coronavirus; mucosal vaccination; route of administration; vaccine.
Figures
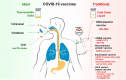

References
-
- Wu F, Zhao S, Yu B, Chen Y, Wang W, Song Z, et al. A new coronavirus associated with human respiratory disease in China. Nature [Internet]. 2020;579(7798):265–9 Available from: http://www.nature.com/articles/s41586-020-2008-3. - PMC - PubMed
-
- Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet [Internet]. 2020; Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673620310229. - PMC - PubMed
-
- Hung IF-N, Lung K-C, Tso EY-K, Liu R, Chung TW-H, Chu M-Y, et al. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet [Internet]. 2020;395(10238):1695–704 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673620310424. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

