Improved Vim targeting for focused ultrasound ablation treatment of essential tremor: A probabilistic and patient-specific approach
- PMID: 32762005
- PMCID: PMC7643361
- DOI: 10.1002/hbm.25157
Improved Vim targeting for focused ultrasound ablation treatment of essential tremor: A probabilistic and patient-specific approach
Abstract
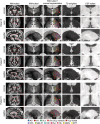
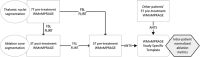
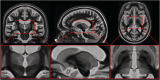
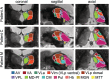
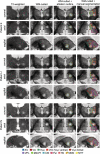
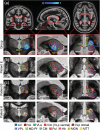
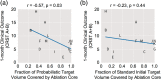
Magnetic resonance-guided focused ultrasound (MRgFUS) ablation of the ventral intermediate (Vim) thalamic nucleus is an incisionless treatment for essential tremor (ET). The standard initial targeting method uses an approximate, atlas-based stereotactic approach. We developed a new patient-specific targeting method to identify an individual's Vim and the optimal MRgFUS target region therein for suppression of tremor. In this retrospective study of 14 ET patients treated with MRgFUS, we investigated the ability of WMnMPRAGE, a highly sensitive and robust sequence for imaging gray matter-white matter contrast, to identify the Vim, FUS ablation, and a clinically efficacious region within the Vim in individual patients. We found that WMnMPRAGE can directly visualize the Vim in ET patients, segmenting this nucleus using manual or automated segmentation capabilities developed by our group. WMnMPRAGE also delineated the ablation's core and penumbra, and showed that all patients' ablation cores lay primarily within their Vim segmentations. We found no significant correlations between standard ablation features (e.g., ablation volume, Vim-ablation overlap) and 1-month post-treatment clinical outcome. We then defined a group-based probabilistic target, which was nonlinearly warped to individual brains; this target was located within the Vim for all patients. The overlaps between this target and patient ablation cores correlated significantly with 1-month clinical outcome (r = -.57, p = .03), in contrast to the standard target (r = -.23, p = .44). We conclude that WMnMPRAGE is a highly sensitive sequence for segmenting Vim and ablation boundaries in individual patients, allowing us to find a novel tremor-associated center within Vim and potentially improving MRgFUS treatment for ET.
Keywords: 7T MRI; MR guided focused ultrasound; Vim; essential tremor; surgical targeting; thalamus; white matter nulled MPRAGE.
© 2020 The Authors. Human Brain Mapping published by Wiley Periodicals LLC.
Conflict of interest statement
P. G. and C. H. H. receive research funding from INSIGHTEC. C. H. H. receives consulting fees from Boston Scientific, Ad‐Tech, and Medtronic. B. K. R. receives research funding from GE Healthcare. The other authors have no other conflicts to declare.
Figures
Similar articles
-
Predicting Post-Operative Side Effects in VIM MRgFUS Based on THalamus Optimized Multi Atlas Segmentation (THOMAS) on White-Matter-Nulled MRI: A Retrospective Study.AJNR Am J Neuroradiol. 2025 Feb 3;46(2):330-340. doi: 10.3174/ajnr.A8448. AJNR Am J Neuroradiol. 2025. PMID: 39730158
-
Magnetic Resonance-Guided Focused Ultrasound for Treatment of Essential Tremor: Ventral Intermediate Nucleus Ablation Alone or Additional Posterior Subthalamic Area Lesioning?Mov Disord Clin Pract. 2024 May;11(5):504-514. doi: 10.1002/mdc3.14005. Epub 2024 Mar 12. Mov Disord Clin Pract. 2024. PMID: 38469997 Free PMC article.
-
MRI and tractography techniques to localize the ventral intermediate nucleus and dentatorubrothalamic tract for deep brain stimulation and MR-guided focused ultrasound: a narrative review and update.Neurosurg Focus. 2020 Jul;49(1):E8. doi: 10.3171/2020.4.FOCUS20170. Neurosurg Focus. 2020. PMID: 32610293 Free PMC article.
-
An experience-based review of HIFU in functional interventional neuroradiology: transcranial MRgFUS thalamotomy for treatment of tremor.Radiol Med. 2020 Sep;125(9):877-886. doi: 10.1007/s11547-020-01186-y. Epub 2020 Apr 7. Radiol Med. 2020. PMID: 32266693 Review.
-
Brain targeting for focused ultrasound essential tremor ablation: proceedings from the 2023 Focused Ultrasound Foundation workshop.Neurosurg Focus. 2024 Sep 1;57(3):E3. doi: 10.3171/2024.6.FOCUS2494. Neurosurg Focus. 2024. PMID: 39217630 Review.
Cited by
-
A detailed analysis of anatomical plausibility of crossed and uncrossed streamline rendition of the dentato-rubro-thalamic tract (DRT(T)) in a commercial stereotactic planning system.Acta Neurochir (Wien). 2021 Oct;163(10):2809-2824. doi: 10.1007/s00701-021-04890-4. Epub 2021 Jun 28. Acta Neurochir (Wien). 2021. PMID: 34181083 Free PMC article.
-
Tremor Severity and Operative Parameters Predict Imbalance in Patients Undergoing Focused Ultrasound Thalamotomy.Mov Disord Clin Pract. 2024 Dec;11(12):1542-1549. doi: 10.1002/mdc3.14237. Epub 2024 Oct 25. Mov Disord Clin Pract. 2024. PMID: 39450579
-
Robust thalamic nuclei segmentation from T1-weighted MRI using polynomial intensity transformation.Brain Struct Funct. 2024 Jun;229(5):1087-1101. doi: 10.1007/s00429-024-02777-5. Epub 2024 Mar 28. Brain Struct Funct. 2024. PMID: 38546872 Free PMC article.
-
Bilateral Focused Ultrasound Thalamotomy for Essential Tremor: Clinical Outcomes Compared to Bilateral Deep Brain Stimulation and Probabilistic Lesion Mapping.Mov Disord. 2025 Jul;40(7):1265-1278. doi: 10.1002/mds.30221. Epub 2025 May 2. Mov Disord. 2025. PMID: 40318052 Free PMC article.
-
Cerebello-Thalamo-Cortical MR Spectroscopy in Patients with Essential Tremor Undergoing MRgFUS Thalamotomy: A Pilot Study.Life (Basel). 2022 Oct 29;12(11):1741. doi: 10.3390/life12111741. Life (Basel). 2022. PMID: 36362896 Free PMC article.
References
-
- Al‐Fatly, B. , Ewert, S. , Kübler, D. , Kroneberg, D. , Horn, A. , & Kühn, A. A. (2019). Connectivity profile of thalamic deep brain stimulation to effectively treat essential tremor. Brain, 142(10), 3086–3098. - PubMed
-
- Anthofer, J. , Steib, K. , Fellner, C. , Lange, M. , Brawanski, A. , & Schlaier, J. (2014). The variability of atlas‐based targets in relation to surrounding major fibre tracts in thalamic deep brain stimulation. Acta Neurochirurgica, 156(8), 1497–1504. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

