Production of Modified Vaccinia Ankara Virus by Intensified Cell Cultures: A Comparison of Platform Technologies for Viral Vector Production
- PMID: 32762152
- PMCID: PMC7435511
- DOI: 10.1002/biot.202000024
Production of Modified Vaccinia Ankara Virus by Intensified Cell Cultures: A Comparison of Platform Technologies for Viral Vector Production
Abstract
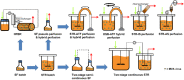
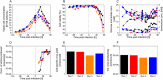
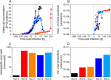
Modified Vaccinia Ankara (MVA) virus is a promising vector for vaccination against various challenging pathogens or the treatment of some types of cancers, requiring a high amount of virions per dose for vaccination and gene therapy. Upstream process intensification combining perfusion technologies, the avian suspension cell line AGE1.CR.pIX and the virus strain MVA-CR19 is an option to obtain very high MVA yields. Here the authors compare different options for cell retention in perfusion mode using conventional stirred-tank bioreactors. Furthermore, the authors study hollow-fiber bioreactors and an orbital-shaken bioreactor in perfusion mode, both available for single-use. Productivity for the virus strain MVA-CR19 is compared to results from batch and continuous production reported in literature. The results demonstrate that cell retention devices are only required to maximize cell concentration but not for continuous harvesting. Using a stirred-tank bioreactor, a perfusion strategy with working volume expansion after virus infection results in the highest yields. Overall, infectious MVA virus titers of 2.1-16.5 × 109 virions/mL are achieved in these intensified processes. Taken together, the study shows a novel perspective on high-yield MVA virus production in conventional bioreactor systems linked to various cell retention devices and addresses options for process intensification including fully single-use perfusion platforms.
Keywords: Modified Vaccinia Ankara virus; continuous production; perfusion cell culture; process intensification.
© 2020 The Authors. Biotechnology Journal published by Wiley-VCH GmbH.
Conflict of interest statement
G.G., F.T., I.B., Y.G., and U.R. declare that they have no conflict of interest. I.J. is an employee of ProBioGen AG which has established the AGE1.CR.pIX cell line and developed the MVA‐CR19 virus strain.
Figures

References
-
- Ozawa S., Clark S., Portnoy A., Grewal S., Brenzel L., Walker D. G., Health Aff. 2016, 35, 199. - PubMed
-
- Bernasconi V., Kristiansen P. A., Whelan M., Román R. G., Bettis A., Yimer S. A., Gurry C., Andersen S. R., Yeskey D., Mandi H., Kumar A., Holst J., Clark C., Cramer J. P., Røttingen J.‐A., Hatchett R., Saville M., Norheim G., Bundesgesundheitsblatt – Gesundheitsforschung – Gesundheitsschutz 2020, 63, 65. - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

