Thyroid Hormone Receptor α Mutations Cause Heart Defects in Zebrafish
- PMID: 32762296
- PMCID: PMC7891307
- DOI: 10.1089/thy.2020.0332
Thyroid Hormone Receptor α Mutations Cause Heart Defects in Zebrafish
Abstract
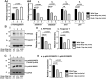
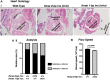
Background: Mutations of thyroid hormone receptor α1 (TRα1) cause resistance to thyroid hormone (RTHα). Patients exhibit growth retardation, delayed bone development, anemia, and bradycardia. By using mouse models of RTHα, much has been learned about the molecular actions of TRα1 mutants that underlie these abnormalities in adults. Using zebrafish models of RTHα that we have recently created, we aimed to understand how TRα1 mutants affect the heart function during this period. Methods: In contrast to human and mice, the thra gene is duplicated, thraa and thrab, in zebrafish. Using CRISPR/Cas9-mediated targeted mutagenesis, we created C-terminal mutations in each of two duplicated thra genes in zebrafish (thraa 8-bp insertion or thrab 1-bp insertion mutations). We recently showed that these mutant fish faithfully recapitulated growth retardation as found in patients and thra mutant mice. In the present study, we used histological analysis, gene expression profiles, confocal fluorescence, and transmission electron microscopy (TEM) to comprehensively analyze the phenotypic characteristics of mutant fish heart during development. Results: We found both a dilated atrium and an abnormally shaped ventricle in adult mutant fish. The retention of red blood cells in the two abnormal heart chambers, and the decreased circulating blood speed and reduced expression of contractile genes indicated weakened contractility in the heart of mutant fish. These abnormalities were detected in mutant fish as early as 35 days postfertilization (juveniles). Furthermore, the expression of genes associated with the sarcomere assembly was suppressed in the heart of mutant fish, resulting in abnormalities of sarcomere organization as revealed by TEM, suggesting that the abnormal sarcomere organization could underlie the bradycardia exhibited in mutant fish. Conclusions: Using a zebrafish model of RTHα, the present study demonstrated for the first time that TRα1 mutants could act to cause abnormal heart structure, weaken contractility, and disrupt sarcomere organization that affect heart functions. These findings provide new insights into the bradycardia found in RTHα patients.
Keywords: blood speed; bradycardia; contractility; heart defects; mutations; zebrafish, TRα.
Conflict of interest statement
No competing financial interests exist.
Figures



References
-
- Koenig RJ 1998. Thyroid hormone receptor coactivators and corepressors. Thyroid 8:703–713 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

